Abstract
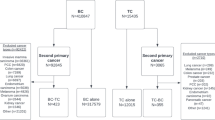
Objective: To evaluate the risk for developing second primary thyroid cancer (TC) following breast cancer (BC) and second primary BC following TC on a nationwide basis. Methods: All BC and TC Jewish females diagnosed in Israel during 1960–1998 were identified through the Israel Cancer Registry. The expected second primaries were calculated using cancer incidence rates stratified by age, country of birth and period of diagnosis among the Jewish population in Israel. Standardized incidence ratios (SIRs) were estimated using Poisson regression. Results: A total of 49,207 breast and 4911 thyroid neoplasms were identified. After the exclusion of concomitant disease (diagnosed within 1 year), 59 and 70 second primaries TC and BC yielded SIRs of 1.34 (95% CI: 1.03, 1.72) and 1.07 (95% CI: 0.84, 1.34), respectively. Younger age and earlier calendar year of first primary diagnosis and shorter follow-up period were associated with increased risk for developing second primary neoplasm. Conclusions: Considering the long latency required for carcinogenesis, excess risk of second primary diagnoses soon after the first cancer, argues against the hypothesis of first primary treatment as an initiator for the second cancer. A detection bias of meticulously followed cancer patients, early exposure to common risk factors or genetic susceptibility of certain subpopulations for both malignancies seem plausible.
Similar content being viewed by others
References
Smyth PP (1993) Thyroid disease and breast cancer. J Endocrinol Invest 16: 396-401.
Giani C, Fierabracci P, Bonacci R, et al. (1996) Relationship between breast cancer and thyroid disease: relevance of autoimmune thyroid disorders in breast malignancy. J Clin Endocrinol Metab 81: 990-994.
Brinton LA, Hoffman DA, Hoover R, Fraumeni Jr JF (1984) Relationship of thyroid disease and use of thyroid supplements to breast cancer risk. J Chronic Dis 37: 877-893.
Zumoff B, O'Connor J, Levin J, Markham N, Strain GW, Fukushima DK (1981) Plasma levels of thyroxine and triiodothyronine in women with breast cancer. Anticancer Res 1: 287-291.
Greenlee RT, Murray T, Bolden S, Wingo PA (2000) Cancer Statistics, 2000. CA Cancer J Clin 50: 7-33.
Breslow NE, Day NE (1987) Fitting models to group data. Statistical methods in cancer research: the design and analysis of cohort studies. IARC Scientific Pub. No. 82, Lyon, France, 119-176.
SAS/STAT Software(1996) Changes and Enhancements, Release 6.11. SAS Institute, Cary, USA.
Ron E, Curtis R, Hoffman DA, Flannery JT (1984) Multiple primary breast and thyroid cancer. Br J Cancer 49: 87-92.
Vassilopoulou-Sellin R, Palmer L, Taylor S, Cooksley CS (1999) Incidence of breast carcinoma in women with thyroid carcinoma. Cancer 85: 696-705.
Li CI, Rossing MA, Voigt LF, Daling JF (2000) Multiple primary breast and thyroid cancers: role of age at diagnosis and cancer treatments(United States). Cancer Causes Control 11: 805-811.
Schenker JG, Levinsky R, Ohel G (1984) Multiple primary malignant neoplasms in breast cancer patients in Israel. Cancer 54: 145-150.
Grossbart-Schwartz A, Ragheb NE, Swanson GM, Satariano WA (1989) Racial and age difference in multiple primary cancersafter breast cancer: a population-based analysis. Breast Cancer Res Treat 14: 215-254.
Buiatti E, Crocetti E, Acciai S, et al. (1997) Incidence of second primary cancers in three Italian population-based cancer registries. Eur J Cancer 33: 1829-1834.
Murakami R, Hiyama T, Hanai A, Fujimoto I (1987) Second primary cancers following female breast cancer in Osaka, Japan-a population-based cohort study. Jpn J Clin Oncol 17: 293-302.
Volk N, Pompe-Kirn V (1997) Second primary cancersin breast cancer patientsin Slovenia. Cancer Causes Control 8: 764-770.
Harvey EB, Brinton LA (1985) Second cancer following cancer of the breast in Connecticut, 1935-1982. Natl Cancer Inst Monogr 68: 99-112.
Ewertz M, Mouridsen H (1985) Second cancer following cancer of the female breast in Denmark 1943-1980. Natl Cancer Inst Monogr 68: 325-329.
Evans HS, Lewis CM, Robinson D, et al. (2001) Incidence of multiple primary cancersin a cohort of women diagnosed with breast cancer in southeast England. Br J Cancer 84: 435-440.
Collaborative Group on Hormonal Factorsin Breast Cancer(2001) Familial breast cancer: collaborative reanalysis of individual data from 52 epidemiological studies including 58,209 women with breast cancer and 101,986 women without the disease. Lancet 358: 1389-1399.
Dahia PL, Marsh DJ, Zheng Z, et al. (1997) Somatic deletionsand mutations in the Cowden disease gene, PTEN, in sporadic thyroid tumors. Cancer Res 57: 4710-4713.
Ron E, Preston DL, Mabuchi K, Thompson DE, Soda M (1994) Cancer Incidence in Atomic Bomb Survivors. Part IV: Comparison of cancer incidence and mortality. Radiat Res 137: S98-S112.
Bhatia S, Robison LL, Oberlin O, Greenberg M, Bunin G, Fossati-Bella Meadows AT (1996) Breast cancer and other second neoplasms after childhood Hodgkin's disease. N Engl J Med 334: 745-751.
de Vathaire F, Hardiman C, Shamsaladin A, et al. (1999) Thyroid carcinomasafter irradiation for a first cancer during childhood. Arch Int Med 159: 2713-2719.
Ron E, Lubin JH, Shore RE, et al. (1995) Thyroid cancer after exposure to external radiation: a pooled analysis of seven studies. Radiat Res 14: 259-277.
Tokunaga M, Land CE, Yamamoto T, Asano M, Tokuoka S, Ezaki H (1987) Incidence of female breast cancer among atomic bomb survivors, Hiroshima and Nagasaki, 1950-1980. Radiat Res 112: 243-272.
Ron E, Modan B, Preston D, Alfandary E, Stovall M, Boice JD Jr (1989) Thyroid neoplasia following low dose radiation in childhood. Radiat Res 120: 516-531.
Baverstock K, Egloff B, Pinchera A, Ruchti C, Williams D (1992) Thyroid cancer after Chernobyl. Nature 359: 21-22.
Li FP, Corkery J, Vawter G, Fine W, Sallan SE (1983) Breast carcinoma after cancer therapy in childhood. Cancer 51: 521-523.
Modan B, Baidatz D, Mart H, Steinitz R, Levin SG (1974) Radiation Induced Head and Neck Tumors. Lancet 1: 277-279.
Sadetzki S, Modan B (1999) Epidemiology as a basis for legislation. How far should epidemiology go? Lancet 353: 2238-2239.
Hall P, Holm LE, Lundell G, et al. (1991) Cancer risks in thyroid cancer patients. Br J Cancer 64: 159-163.
Brinton LA, Schairer C, Hoover RN, Fraumeni JF, Jr (1988) Menstrual factors and risk of breast cancer. Cancer Invest 6: 245-254.
Pike MC, Henderson BE, Casagrande JT (1981) The epidemiology of breast cancer as it relates to menarche, pregnancy and menopause. In: Pike MC, Siiteri PJ, Welsch CW, eds. Hormones and Breast Cancer. (Banbury Report No. 8). Cold Spring Harbor, NY: Cold Spring Harbor Laboratory, pp. 3-18.
Ewertz M, Duffy SW (1988) Risk of breast cancer in relation to reproductive factor in Denmark. Br J Cancer 58: 99-104.
Collaborative Group on Hormonal Factorsin Breast Cancer(1996) Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53,297 women with breast cancer and 100,239 women without breast cancer from 54 epidemiological studies. Lancet 347: 1713-1727.
Collaborative Group on Hormonal Factorsin Breast Cancer(1997) Breast cancer and hormonal contraceptives: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 without breast cancer. Lancet 350: 1047-1059.
Negri E, Dal Maso L, Ron E, et al. (1999) A pooled analysis of case-control studies of thyroid cancer. II. Menstrual and reproductive factors. III. Oral contraceptives, menopausal replacement therapy and other female hormones. Cancer Causes Control 10: 143-166.
La Vecchia C, Ron E, Franceschi S, et al. (1999) A pooled analysis of case-controls studies of thyroid cancer. III. Oral contraceptives, menopausal replacement therapy and other female hormones. Cancer Causes Control 10: 157-166.
Goldman MB (1990) Thyroid disease and breast cancer. Epi Rev 12: 16-28.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sadetzki, S., Calderon-Margalit, R., Peretz, C. et al. Second primary breast and thyroid cancers (Israel). Cancer Causes Control 14, 367–375 (2003). https://doi.org/10.1023/A:1023908509928
Issue Date:
DOI: https://doi.org/10.1023/A:1023908509928