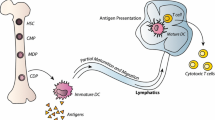
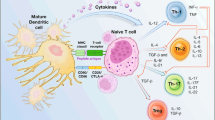
Abstract
Despite advancements in therapeutic regimens, the prognosis remains poor for patients with malignant gliomas. Specificity has been an elusive goal for current modalities, but immunotherapy has emerged as a potential means of designing more tumor-specific treatments. Dendritic cells (DC) are the specialized antigen presenting cells of the immune system and have served now as a platform for therapeutic immunizations against such cancers as lymphoma, multiple myeloma, melanoma, prostate cancer, renal cell carcinoma, non-small cell lung carcinoma, colon cancer, and even malignant gliomas. DC-based immunizations offer a number of advantages over traditional immunotherapeutic approaches to brain tumors, approaches that have proved promising despite concerns over central nervous system immune privilege and glioma-mediated immunosuppression. The future success of clinical trials will depend on the optimization and standardizing of procedures for DC generation, loading, and administration.
Similar content being viewed by others
References
Dropcho EJ: Novel chemotherapeutic approaches to brain tumors. Hematol/Oncol Clin North Am 15: 1027–1052, 2001
Choucair AK, Levin VA, Gutin PH, Davis RL, Silver P, Edwards MS, Wilson CB: Development of multiple lesions during radiation therapy and chemotherapy in patients with gliomas. J Neurosurg 65: 654–658, 1986
Dandy WE: Removal of right cerebral hemisphere for certian tumors with hemiplegia. J Am Med Assoc 90: 823–825, 1928
Curran WJ Jr, Scott CB, Horton J, Nelson JS, Weinstein AS, Fischbach AJ, Chang CH, Rotman M, Asbell SO, Krisch RE: Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst 85: 704–710, 1993
Coley WB: The Treatment of Inoperable Sarcoma by Bacterial Toxins (the Mixed Toxins of the Streptococcus erysipelas and the Bacillus prodigiosus). John Bale, Sons, & Danielson, London: 1909
van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, Knuth A, Boon T: A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science 254: 1643–1647, 1991
Zinkernagel RM, Doherty PC: MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restrictionspecificity, function, and responsiveness. Adv Immunol 27: 51–177, 1979
Van Pel A, Boon T: Protection against a nonimmunogenic mouse leukemia by an immunogenic variant obtained by mutagenesis. Proc Natl Acad Sci USA 79: 4718–4722, 1982
Tanaka K, Isselbacher KJ, Khoury G, Jay G: Reversal of oncogenesis by the expression of a major histocompatibility complex class I gene. Science 228: 26–30, 1985
Tanaka K, Hayashi H, Hamada C, Khoury G, Jay G: Expression of major histocompatibility complex class I antigens as a strategy for the potentiation of immune recognition of tumor cells. Proc Natl Acad Sci USA 83: 8723–8727, 1986
Braciale TJ, Yap KL: Role of viral infectivity in the induction of influenza virus-specific cytotoxic T cells. J Exp Med 147: 1236–1252, 1978
Steinman RM, Cohn ZA: Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med/J Exp Med 137: 1142–1162, 1973
Steinman RM: The dendritic cell system and its role in immunogenicity. Ann Rev Immunol 9: 271–296, 1991
Bevan MJ: Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J Exp Med 143: 1283–1288, 1976
Matzinger P, Bevan MJ: Induction of H-2-restricted cytotoxic T cells: in vivo induction has the appearance of being unrestricted. Cell Immunol 33: 92–100, 1977
Steinman RM, Gutchinov B, Witmer MD, Nussenzweig MC: Dendritic cells are the principal stimulators of the primary mixed leukocyte reaction in mice. J Exp Med 157: 613–627, 1983
Inaba K, Steinman RM: Resting and sensitized T lymphocytes exhibit distinct stimulatory (antigen-presenting cell) requirements for growth and lymphokine release. J Exp Med/J Exp Med 160: 1717–1735, 1984
Inaba K, Steinman RM: Protein-specific helper Tlymphocyte formation initiated by dendritic cells. Science 229: 475–479, 1985
Inaba K, Young JW, Steinman RM: Direct activation of CD8+ cytotoxic T lymphocytes by dendritic cells. J Exp Med 166: 182–194, 1987
Macatonia SE, Taylor PM, Knight SC, Askonas BA: Primary stimulation by dendritic cells induces antiviral proliferative and cytotoxic T cell responses in vitro. J Exp Med/J Exp Med 169: 1255–1264, 1989
Inaba K, Metlay JP, Crowley MT, Steinman RM: Dendritic cells pulsed with protein antigens in vitro can prime antigen-specific, MHC-restricted T cells in situ. J Exp Med/J Exp Med 172: 631–640, 1990
Takahashi H, Nakagawa Y, Yokomuro K, Berzofsky JA: Induction of CD8+ cytotoxic T lymphocytes by immunization with syngeneic irradiated HIV-1 envelope derived peptide-pulsed dendritic cells. Int Immunol 5: 849–857, 1993
Inaba K, Inaba M, Deguchi M, Hagi K, Yasumizu R, Ikehara S, Muramatsu S, Steinman RM: Granulocytes, macrophages, and dendritic cells arise from a common major histocompatibility complex class II-negative progenitor in mouse bone marrow. Proc Natl Acad Sci USA 90: 3038–3042, 1993
Harshyne LA, Watkins SC, Gambotto A, Barratt-Boyes SM: Dendritic cells acquire antigens from live cells for cross-presentation to CTL. J Immunol 166: 3717–3723, 2001
Guermonprez P, Valladeau J, Zitvogel L, Thery C, Amigorena S: Antigen presentation and T cell stimulation by dendritic cells. Ann Rev Immunol 20: 621–667, 2002
Matzinger P: Tolerance, danger, and the extended family. Ann Rev Immunol 12: 991–1045, 1994
Gallucci S, Matzinger P: Danger signals: SOS to the immune system. Curr Opin Immunol 13: 114–119, 2001
Nestle FO, Banchereau J, Hart D: Dendritic cells: on the move from bench to bedside. Nature Med 7: 761–765, 2001
Sallusto F, Lanzavecchia A: Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med 179: 1109–1118, 1994
Sallusto F, Cella M, Danieli C, Lanzavecchia A: Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med 182: 389–400, 1995
Banchereau J, Steinman RM: Dendritic cells and the control of immunity. Nature 392: 245–252, 1998
Lanier LL, O'Fallon S, Somoza C, Phillips JH, Linsley PS, Okumura K, Ito D, Azuma M: CD80 (B7) and CD86 (B70) provide similar costimulatory signals for T cell proliferation, cytokine production, and generation of CTL. J Immunol 154: 97–105, 1995
Cella M, Engering A, Pinet V, Pieters J, Lanzavecchia A: Inflammatory stimuli induce accumulation ofMHCclass II complexes on dendritic cells. Nature 388: 782–787, 1997
Dieu MC, Vanbervliet B, Vicari A, Bridon JM, Oldham E, Ait-Yahia S, Briere F, Zlotnik A, Lebecque S, Caux C: Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med 188: 373–386, 1998
Ingulli E, Mondino A, Khoruts A, Jenkins MK: In vivo detection of dendritic cell antigen presentation to CD4(+) T cells. J Exp Med 185: 2133–2141, 1997
Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G: Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help viaAPC activation. J Exp Med 184: 747–752, 1996
Bennett SR, Carbone FR, Karamalis F, Miller JF, Heath WR: Induction of a CD8+ cytotoxic T lymphocyte response by cross-priming requires cognate CD4+ T cell help. J Exp Med/J Exp Med 186: 65–70, 1997
Huang AY, Golumbek P, Ahmadzadeh M, Jaffee E, Pardoll D, Levitsky H: Role of bone marrow-derived cells in presenting MHC class I-restricted tumor antigens. Science 264: 961–965, 1994
Grabbe S, Beissert S, Schwarz T, Granstein RD: Dendritic cells as initiators of tumor immune responses: a possible strategy for tumor immunotherapy? [see comments.]. Immunol Today 16: 117–121, 1995
Ambe K, Mori M, Enjoji M: S-100 protein-positive dendritic cells in colorectal adenocarcinomas. Distribution and relation to the clinical prognosis. Cancer 63: 496–503, 1989
Tsujitani S, Kakeji Y, Watanabe A, Kohnoe S, Maehara Y, Sugimachi K: Infiltration of dendritic cells in relation to tumor invasion and lymph node metastasis in human gastric cancer. Cancer 66: 2012–2016, 1990
Alcalay J, Kripke ML: Antigen-presenting activity of draining lymph node cells from mice painted with a contact allergen during ultraviolet carcinogenesis. J Immunol 146: 1717–1721, 1991
Watson GA, Lopez DM: Aberrant antigen presentation by macrophages from tumor-bearing mice is involved in the down-regulation of their T cell responses. J Immunol 155: 3124–3134, 1995
Toriyama K, Wen DR, Paul E, Cochran AJ: Variations in the distribution, frequency, and phenotype of Langerhans cells during the evolution of malignant melanoma of the skin. J Invest Dermatol 100: 269S–273S, 1993
Schuler G, Steinman RM: Dendritic cells as adjuvants for immune-mediated resistance to tumors. J Exp Med/J Exp Med 186: 1183–1187, 1997
Gabrilovich DI, Chen HL, Girgis KR, Cunningham HT, Meny GM, Nadaf S, Kavanaugh D, Carbone DP: Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nature Med 2: 1096–1103, 1996
Chen Q, Daniel V, Maher DW, Hersey P: Production of IL-10 by melanoma cells: examination of its role in immunosuppression mediated by melanoma. International J Cancer 56: 755–760, 1994
Grabbe S, Bruvers S, Gallo RL, Knisely TL, Nazareno R, Granstein RD: Tumor antigen presentation by murine epidermal cells. J Immunol 146: 3656–3661, 1991
Flamand V, Sornasse T, Thielemans K, Demanet C, Bakkus M, Bazin H, Tielemans F, Leo O, Urbain J, Moser M: Murine dendritic cells pulsed in vitro with tumor antigen induce tumor resistance in vivo. Euro J Immunol 24: 605–610, 1994
Cohen PJ, Cohen PA, Rosenberg SA, Katz SI, Mule JJ: Murine epidermal Langerhans cells and splenic dendritic cells present tumor-associated antigens to primed T cells. Euro J Immunol 24: 315–319, 1994
Shimizu J, Suda T, Yoshioka T, Kosugi A, Fujiwara H, Hamaoka T: Induction of tumor-specific in vivo protective immunity by immunization with tumor antigen-pulsed antigen-presenting cells. J Immunol 142: 1053–1059, 1989
Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM: Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colonystimulating factor. J Exp Med 176: 1693–1702, 1992
Porgador A, Gilboa E: Bone marrow-generated dendritic cells pulsed with a class I-restricted peptide are potent inducers of cytotoxic T lymphocytes. J Exp Med/J Exp Med 182: 255–260, 1995
Mayordomo JI, Zorina T, Storkus WJ, Zitvogel L, Celluzzi C, Falo LD, Melief CJ, Ildstad ST, Kast WM, Deleo AB: Bone marrow-derived dendritic cells pulsed with synthetic tumour peptides elicit protective and therapeutic antitumour immunity. Nature Med 1: 1297–1302, 1995
Celluzzi CM, Mayordomo JI, Storkus WJ, Lotze MT, Falo LD Jr: Peptide-pulsed dendritic cells induce antigenspecific CTL-mediated protective tumor immunity. J Exp Med 183: 283–287, 1996
Porgador A, Snyder D, Gilboa E: Induction of antitumor immunity using bone marrow-generated dendritic cells. J Immunol 156: 2918–2926, 1996
Paglia P, Chiodoni C, Rodolfo M, Colombo MP: Murine dendritic cells loaded in vitro with soluble protein prime cytotoxic T lymphocytes against tumor antigen in vivo. J Exp Med 183: 317–322, 1996
Zitvogel L, Mayordomo JI, Tjandrawan T, DeLeo AB, Clarke MR, Lotze MT, Storkus WJ: Therapy of murine tumors with tumor peptide-pulsed dendritic cells: dependence on T cells, B7 costimulation, and T helper cell 1-associated cytokines. J Exp Med/J Exp Med 183: 87–97, 1996
Faiola B, Doyle C, Gilboa E, Nair S: Influence of CD4 T cells and the source of major histocompatibility complex class II-restricted peptides on cytotoxic T-cell priming by dendritic cells. Immunology 105: 47–55, 2002
Mackensen A, Drager R, Schlesier M, Mertelsmann R, Lindemann A: Presence of IgE antibodies to bovine serum albumin in a patient developing anaphylaxis after vaccination with human peptide-pulsed dendritic cells. Cancer Immunol Immunother 49: 152–156, 2000
Maloney DG, Kaminski MS, Burowski D, Haimovich J, Levy R: Monoclonal anti-idiotype antibodies against the murine B cell lymphoma 38C13: characterization and use as probes for the biology of the tumor in vivo and in vitro. Hybridoma 4: 191–209, 1985
Romani N, Gruner S, Brang D, Kampgen E, Lenz A, Trockenbacher B, Konwalinka G, Fritsch PO, Steinman RM, Schuler G: Proliferating dendritic cell progenitors in human blood. J Exp Med 180: 83–93, 1994
Hsu FJ, Benike C, Fagnoni F, Liles TM, Czerwinski D, Taidi B, Engleman EG, Levy R: Vaccination of patients with B-cell lymphoma using autologous antigen-pulsed dendritic cells. Nature Med 2: 52–58, 1996
Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D: Vaccination of melanoma patients with peptide-or tumor lysate-pulsed dendritic cells. Nature Med 4: 328–332, 1998
Thurner B, Haendle I, Roder C, Dieckmann D, Keikavoussi P, Jonuleit H, Bender A, Maczek C, Schreiner D, von den Driesch P, Brocker EB, Steinman RM, Enk A, Kampgen E, Schuler G:Vaccination with mage-3A1 peptide-pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J Exp Med/J Exp Med 190: 1669–1678, 1999
Mackensen A, Herbst B, Chen JL, Kohler G, Noppen C, Herr W, Spagnoli GC, Cerundolo V, Lindemann A: Phase I study in melanoma patients of a vaccine with peptidepulsed dendritic cells generated in vitro from CD34(+) hematopoietic progenitor cells. Int J Cancer 86: 385–392, 2000
Murphy GP, Tjoa BA, Simmons SJ, Ragde H, Rogers M, Elgamal A, Kenny GM, Troychak MJ, Salgaller ML, Boynton AL: Phase II prostate cancer vaccine trial: report of a study involving 37 patients with disease recurrence following primary treatment. Prostate 39: 54–59, 1999
Fong L, Brockstedt D, Benike C, Wu L, Engleman EG: Dendritic cells injected via different routes induce immunity in cancer patients. J Immunol 166: 4254–4259, 2001
Holtl L, Rieser C, Papesh C, Ramoner R, Herold M, Klocker H, Radmayr C, Stenzl A, Bartsch G, Thurnher M: Cellular and humoral immune responses in patients with metastatic renal cell carcinoma after vaccination with antigen pulsed dendritic cells. J Urol 161: 777–782, 1999
Kugler A, Stuhler G, Walden P, Zoller G, Zobywalski A, Brossart P, Trefzer U, Ullrich S, Muller CA, Becker V, Gross AJ, Hemmerlein B, Kanz L, Muller GA, Ringert RH: Regression of human metastatic renal cell carcinoma after vaccination with tumor cell-dendritic cell hybrids. Nature Med 6: 332–336, 2000
Fong L, Hou Y, Rivas A, Benike C, Yuen A, Fisher GA, Davis MM, Engleman EG: Altered peptide ligand vaccination with Flt3 ligand expanded dendritic cells for tumor immunotherapy. Proc Natl Acad Sci USA 98: 8809–8814, 2001
Morse MA, Nair S, Fernandez-Casal M, Deng Y, St Peter M, Williams R, Hobeika A, Mosca P, Clay T, Cumming RI, Fisher E, Clavien P, Proia AD, Niedzwiecki D, Caron D, Lyerly HK: Preoperative mobilization of circulating dendritic cells by Flt3 ligand administration to patients with metastatic colon cancer. J Clin Oncol 18: 3883–3893, 2000
Dhodapkar MV, Steinman RM, Sapp M, Desai H, Fossella C, Krasovsky J, Donahoe SM, Dunbar PR, Cerundolo V, Nixon DF, Bhardwaj N: Rapid generation of broad T-cell immunity in humans after a single injection of mature dendritic cells. J Clin Invest 104: 173–180, 1999
Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N: Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med 193: 233–238, 2001
Heimberger AB, Archer GE, Crotty LE, McLendon RE, Friedman AH, Friedman HS, Bigner DD, Sampson JH: Dendritic cells pulsed with a tumor-specific peptide induce long-lasting immunity and are effective against murine intracerebral melanoma. Neurosurgery 50: 158–164; Discussion 164–156, 2002
Heimberger AB, Crotty LE, Archer GE, McLendon RE, Friedman A, Dranoff G, Bigner DD, Sampson JH: Bone marrow-derived dendritic cells pulsed with tumor homogenate induce immunity against syngeneic intracerebral glioma. J Neuroimmunol 103: 16–25, 2000
Okada H, Tahara H, Shurin MR, Attanucci J, Giezeman-Smits KM, Fellows WK, Lotze MT, Chambers WH, Bozik ME: Bone marrow-derived dendritic cells pulsed with a tumor-specific peptide elicit effective anti-tumor immunity against intracranial neoplasms. Int J Cancer 78: 196–201, 1998
Ashley DM, Faiola B, Nair S, Hale LP, Bigner DD, Gilboa E: Bone marrow-generated dendritic cells pulsed with tumor extracts or tumor RNA induce antitumor immunity against central nervous system tumors. J Exp Med 186: 1177–1182, 1997
Liau LM, Black KL, Prins RM, Sykes SN, DiPatre PL, Cloughesy TF, Becker DP, Bronstein JM: Treatment of intracranial gliomas with bone marrow-derived dendritic cells pulsed with tumor antigens. J Neurosurg 90: 1115–1124, 1999
Ni HT, Spellman SR, Jean WC, Hall WA, Low WC: Immunization with dendritic cells pulsed with tumor extract increases survival of mice bearing intracranial gliomas. J Neuro-Oncology 51: 1–9, 2001
Yamanaka R, Zullo SA, Tanaka R, Blaese M, Xanthopoulos KG: Enhancement of antitumor immune response in glioma models in mice by genetically modified dendritic cells pulsed with Semliki forest virus-mediated complementary DNA. J Neurosurg 94: 474–481, 2001
Wong AJ, Ruppert JM, Bigner SH, Grzeschik CH, Humphrey PA, Bigner DS, Vogelstein B: Structural alterations of the epidermal growth factor receptor gene in human gliomas. Proc Natl Acad Sci USA 89: 2965–2969, 1992
Wikstrand CJ, McLendon RE, Friedman AH, Bigner DD: Cell surface localization and density of the tumorassociated variant of the epidermal growth factor receptor, EGFRvIII. Cancer Research 57: 4130–4140, 1997
Sornasse T, Flamand, V, De Becker G, Bazin H, Tielemans F, Thielemans K, Urbain J, Leo O, Moser M: Antigen-pulsed dendritic cells can efficiently induce an antibody response in vivo. J Exp Med/J Exp Med 175: 15–21, 1992
Yu JS, Wheeler CJ, Zeltzer PM, Ying H, Finger DN, Lee PK, Yong WH, Incardona F, Thompson RC, Riedinger MS, Zhang W, Prins RM, Black KL: Vaccination of malignant glioma patients with peptide-pulsed dendritic cells elicits systemic cytotoxicity and intracranial T-cell infiltration. Cancer Res 61: 842–847, 2001
Morse MA, Coleman RE, Akabani G, Niehaus N, Coleman D, Lyerly HK: Migration of human dendritic cells after injection in patients with metastatic malignancies. Cancer Res 59: 56–58, 1999
Barratt-Boyes SM, Zimmer MI, Harshyne LA, Meyer EM, Watkins SC, Capuano S, 3rd, Murphey-Corb M, Falo LD Jr, Donnenberg AD: Maturation and trafficking of monocytederived dendritic cells in monkeys: implications for dendritic cell-based vaccines. J Immunol 164: 2487–2495, 2000
Kalinski P, Vieira PL, Schuitemaker JH, de Jong EC, Kapsenberg ML: Prostaglandin E(2) is a selective inducer of interleukin-12 p40 (IL-12p40) production and an inhibitor of bioactive IL-12p70 heterodimer. Blood 97: 3466–3469, 2001
Liau LM, Salgaller ML, Kiertscher SM, Cloughesy TF, Mischel PS, Martin NA, Becker DP, Roth MD: Dendritic cell vaccine presenting eluted tumor antigens for treatment of patients with glioblastoma multiforme: Interim Phase I trial results of DCVax[tm]-Brainelu. In: Proceedings of the American Association for Cancer Research, San Francisco, CA, 2002
Sloan AE, Parajuli P, Mathupala S: DC-tumor cell fusion for induction of tumor-specific T-cell response against malignant brain tumors: Comparison with DC pulsed with total tumor RNA or tumor lysate. In: Proceedings of the American Association for Cancer Research, San Francisco, CA, 2002
Bigner DD, Pitts OM, Wikstrand CJ: Induction of lethal Exp allergic encephalomyelitis in nonhuman primates and guinea pigs with human glioblastoma multiforme tissue. J Neurosurg 55: 32–42, 1981
Bloom MB, Perry-Lalley D, Robbins PF, Li Y, el-Gamil M, Rosenberg SA, Yang JC: Identification of tyrosinaserelated protein 2 as a tumor rejection antigen for the B16 melanoma. J Exp Med 185: 453–459, 1997
Ropke M, Hald J, Guldberg P, Zeuthen J, Norgaard L, Fugger L, Svejgaard A, Van der Burg S, Nijman HW, Melief CJ, Claesson MH: Spontaneous human squamous cell carcinomas are killed by a human cytotoxic T lymphocyte clone recognizing a wild-type p53-derived peptide. In: Proceedings of the National Academy of Sciences of the United States of America, 93: 14704–14707, 1996
Ogg GS, Rod Dunbar P, Romero P, Chen JL, Cerundolo V: High frequency of skin-homing melanocyte-specific cytotoxic T lymphocytes in autoimmune vitiligo. J Exp Med 188: 1203–1208, 1998
Rosenberg SA, White DE: Vitiligo in patients with melanoma: normal tissue antigens can be targets for cancer immunotherapy. J Immunother Emphasis Tumor Immunol 19: 81–84, 1996
Dittel BN, Visintin I, Merchant RM, Janeway CA Jr: Presentation of the self antigen myelin basic protein by dendritic cells leads to Exp autoimmune encephalomyelitis. J Immunol 163: 32–39, 1999
Ludewig B, Ochsenbein AF, Odermatt B, Paulin D, Hengartner H, Zinkernagel RM: Immunotherapy with dendritic cells directed against tumor antigens shared with normal host cells results in severe autoimmune disease. J Exp Med 191: 795–804, 2000
Ludewig B, Odermatt B, Landmann S, Hengartner H, Zinkernagel RM: Dendritic cells induce autoimmune diabetes and maintain disease via de novo formation of local lymphoid tissue. J Exp Med 188: 1493–1501, 1998
Shortman K, Liu YJ: Mouse and human dendritic cell subtypes. Nature Rev Immunol 2: 151–161, 2002
Kronin V, Winkel K, Suss G, Kelso A, Heath W, Kirberg J, von Boehmer H, Shortman K: A subclass of dendritic cells regulates the response of naive CD8 T cells by limiting their IL-2 production. J Immunol 157: 3819–3827, 1996
Suss G, Shortman, K: A subclass of dendritic cells kills CD4 T cells via Fas/Fas-ligand-induced apoptosis. J Exp Med 183: 1789–1796, 1996
Ruedl C, Bachmann MF: CTL priming by CD8(+) and CD8(+) dendritic cells in vivo. Eur J Immunol 29: 3762–3767, 1999
Moser M, Murphy KM: Dendritic cell regulation of TH1– TH2 development. Nature Immunol 1: 199–205, 2000
Maldonado-Lopez R, De Smedt T, Michel P, Godfroid J, Pajak B, Heirman C, Thielemans K, Leo O, Urbain J, Moser M: CD8alpha+ and CD8alpha– subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J Exp Med 189: 587–592, 1999
Pulendran B, Smith JL, Caspary G, Brasel K, Pettit D, Maraskovsky E, Maliszewski CR: Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc Natl Acad Sci USA 96: 1036–1041, 1999
Smith AL, de St Groth BF: Antigen-pulsed CD8alpha+ dendritic cells generate an immune response after subcutaneous injection without homing to the draining lymph node. J Exp Med/J Exp Med 189: 593–598, 1999
den Haan JM, Lehar SM, Bevan MJ: CD8(+) but not CD8(–) dendritic cells cross-prime cytotoxic T cells in vivo. J Exp Med/J Exp Med 192: 1685–1696, 2000
Pooley JL, Heath WR, Shortman K: Cutting edge: intravenous soluble antigen is presented to CD4 T cells by CD8– dendritic cells, but cross-presented to CD8 T cells byCD8+dendritic cells. J Immunol 166: 5327–5330, 2001
Zitvogel L, Angevin E, Tursz T: Dendritic cell-based immunotherapy of cancer. Ann Oncol 11: 199–205, 2000
Mortarini R, Anichini A, Di Nicola M, Siena S, Bregni M, Belli F, Molla A, Gianni AM, Parmiani G: Autologous dendritic cells derived from CD34+ progenitors and from monocytes are not functionally equivalent antigen-presenting cells in the induction of melan-A/Mart-1(27–35)-specific CTLs from peripheral blood lymphocytes of melanoma patients with low frequency of CTL precursors. Cancer Res 57: 5534–5541, 1997
Randolph GJ, Beaulieu S, Lebecque S, Steinman RM, Muller WA: Differentiation of monocytes into dendritic cells in a model of transendothelial trafficking. Science 282: 480–483, 1998
Randolph GJ, Inaba K, Robbiani DF, Steinman RM, Muller WA: Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity 11: 753–761, 1999
Maraskovsky E, Daro E, Roux E, Teepe M, Maliszewski CR, Hoek J, Caron D, Lebsack ME, McKenna HJ: In vivo generation of human dendritic cell subsets by Flt3 ligand. Blood 96: 878–884, 2000
Maraskovsky E, Brasel K, Teepe M, Roux ER, Lyman SD, Shortman K, McKenna HJ: Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J Exp Med 184: 1953–1962, 1996
Brasel K, De Smedt T, Smith JL, Maliszewski CR: Generation of murine dendritic cells from flt3-ligandsupplemented bone marrow cultures. Blood 96: 3029–3039, 2000
Nair SK, Hull S, Coleman D, Gilboa E, Lyerly HK, Morse MA: Induction of carcinoembryonic antigen (CEA)-specific cytotoxic T-lymphocyte responses in vitro using autologous dendritic cells loaded with CEA peptide or CEA RNA in patients with metastatic malignancies expressing CEA. Int J Cancer 82: 121–124, 1999
Morse MA, Deng Y, Coleman D, Hull S, Kitrell-Fisher E, Nair S, Schlom J, Ryback ME, Lyerly HK: A Phase I study of active immunotherapy with carcinoembryonic antigen peptide (CAP-1)-pulsed, autologous human cultured dendritic cells in patients with metastatic malignancies expressing carcinoembryonic antigen. Clin Cancer Res 5: 1331–1338, 1999
Nair SK, Heiser A, Boczkowski D, Majumdar A, Naoe M, Lebkowski JS, Vieweg J, Gilboa E: Induction of cytotoxic T cell responses and tumor immunity against unrelated tumors using telomerase reverse transcriptase RNA transfected dendritic cells. Nature Med 6: 1011–1017, 2000
Binder RJ, Han DK, Srivastava PK: CD91: a receptor for heat shock protein gp96. Nature Immunol 1: 151–155, 2000
Gallucci S, Lolkema M, Matzinger P: Natural adjuvants: endogenous activators of dendritic cells. Nature Med 5: 1249–1255, 1999
Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj, N: Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med 191: 423–434, 2000
Boczkowski D, Nair SK, Nam JH, Lyerly HK, Gilboa E: Induction of tumor immunity and cytotoxic T lymphocyte responses using dendritic cells transfected with messenger RNA amplified from tumor cells. Cancer Res 60: 1028–1034, 2000
Mitchell DA, Nair SK: RNA-transfected dendritic cells in cancer immunotherapy. J Clin Invest 106: 1065–1069, 2000
Boczkowski D, Nair SK, Snyder D, Gilboa E: Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J Exp Med 184: 465–472, 1996
Lal A, Sui I, Riggins GJ: Serial analysis of gene expression: probing transcriptomes for molecular targets. Curr Opin Mole Ther 1: 720–726, 1999
Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S: Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nature Med 4: 594–600, 1998
Thery C, Regnault A, Garin J, Wolfers J, Zitvogel L, Ricciardi-Castagnoli P, Raposo G, Amigorena S: Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J Cell Biol 147: 599–610, 1999
Wolfers J, Lozier A, Raposo G, Regnault A, Thery C, Masurier C, Flament C, Pouzieux S, Faure F, Tursz T, Angevin E, Amigorena S, Zitvogel L: Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nature Med 7: 297–303, 2001
Specht JM, Wang G, Do MT, Lam JS, Royal RE, Reeves ME, Rosenberg SA, Hwu P: Dendritic cells retrovirally transduced with a model antigen gene are therapeutically effective against established pulmonary metastases. J Exp Med/J Exp Med 186: 1213–1221, 1997
Song W, Kong HL, Carpenter H, Torii H, Granstein R, Rafii S, Moore MA, Crystal RG: Dendritic cells genetically modified with an adenovirus vector encoding the cDNA for a model antigen induce protective and therapeutic antitumor immunity. J Exp Med/J Exp Med 186: 1247–1256, 1997
Nair SK, Morse M, Boczkowski D, Cumming RI, Vasovic L, Gilboa E, Lyerly HK: Induction of tumorspecific cytotoxic T lymphocytes in cancer patients by autologous tumor RNA-transfected dendritic cells. Ann Surg 235: 540–549, 2002
Heiser A, Coleman D, Dannull J, Yancey D, Maurice MA, Lallas CD, Dahm P, Niedzwiecki D, Gilboa E, Vieweg J: Autologous dendritic cells transfected with prostatespecific antigen RNA stimulate CTL responses against metastatic prostate tumors. J Clin Invest 109: 409–417, 2002
Gilboa E, Nair SK, Lyerly HK: Immunotherapy of cancer with dendritic-cell-based vaccines. Cancer Immunol Immunother 46: 82–87, 1998
Berard F, Blanco P, Davoust J, Neidhart-Berard EM, Nouri-Shirazi M, Taquet N, Rimoldi D, Cerottini JC, Banchereau J, Palucka AK: Cross-priming of naive CD8 T cells against melanoma antigens using dendritic cells loaded with killed allogeneic melanoma cells. J Exp Med 192: 1535–1544, 2000
Nouri-Shirazi M, Banchereau J, Bell D, Burkeholder S, Kraus ET, Davoust J, Palucka KA: Dendritic cells capture killed tumor cells and present their antigens to elicit tumorspecific immune responses. J Immunol 165: 3797–3803, 2000
Regnault A, Lankar D, Lacabanne V, Rodriguez A, Thery C, Rescigno M, Saito T, Verbeek S, Bonnerot C, Ricciardi-Castagnoli P, Amigorena S: Fcgamma receptormediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J Exp Med 189: 371–380, 1999
Binder RJ, Blachere NE, Srivastava PK: Heat shock protein-chaperoned peptides but not free peptides introduced into the cytosol are presented efficiently by major histocompatibility complex I molecules. J Biol Chem 276: 17163–17171, 2001
Singh-Jasuja H, Toes RE, Spee P, Munz C, Hilf N, Schoenberger SP, Ricciardi-Castagnoli P, Neefjes J, Rammensee HG, Arnold-Schild D, Schild H: Crosspresentation of glycoprotein 96-associated antigens on major histocompatibility complex class I molecules requires receptor-mediated endocytosis. J Exp Med 191: 1965–1974, 2000
Morse MA, Zhou LJ, Tedder TF, Lyerly HK, Smith C: Generation of dendritic cells in vitro from peripheral blood mononuclear cells with granulocyte-macrophage-colonystimulating factor, interleukin-4, and tumor necrosis factor-alpha for use in cancer immunotherapy. Ann Surg 226: 6–16, 1997
Mosca PJ, Hobeika AC, Clay TM, Nair SK, Thomas EK, Morse MA, Lyerly HK: A subset of human monocytederived dendritic cells expresses high levels of interleukin-12 in response to combined CD40 ligand and interferon-gamma treatment. Blood 96: 3499–3504, 2000
Macatonia SE, Hosken NA, Litton M, Vieira P, Hsieh CS, Culpepper JA, Wysocka M, Trinchieri G, Murphy KM, O'Garra A: Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J Immunol 154: 5071–5079, 1995
Morse MA, Lyerly HK, Gilboa E, Thomas E, Nair SK: Optimization of the sequence of antigen loading and CD40-ligand-induced maturation of dendritic cells. Cancer Res 58: 2965–2968, 1998
Eggert AA, Schreurs MW, Boerman OC, Oyen WJ, de Boer AJ, Punt CJ, Figdor CG, Adema GJ: Biodistribution and vaccine efficiency of murine dendritic cells are dependent on the route of administration. Cancer Res 59: 3340–3345, 1999
Aloisi F, Ria F, Columba-Cabezas S, Hess H, Penna G, Adorini L: Relative efficiency of microglia, astrocytes, dendritic cells and B cells in naive CD4+ T cell priming and Th1/Th2 cell restimulation. Eur J Immunol 29: 2705–2714, 1999
Serot JM, Foliguet B, Bene MC, Faure GC: Ultrastructural and immunohistological evidence for dendritic-like cells within human choroid plexus epithelium. Neuroreport 8: 1995–1998, 1997
McMenamin PG: Distribution and phenotype of dendritic cells and resident tissue macrophages in the dura mater, leptomeninges, and choroid plexus of the rat brain as demonstrated in wholemount preparations. J Comp Neurol 405: 553–562, 1999
Fischer HG, Reichmann G: Brain dendritic cells and macrophages/microglia in central nervous system inflammation. J Immunol 166: 2717–2726, 2001
Fischer HG, Bonifas U, Reichmann G: Phenotype and functions of brain dendritic cells emerging during chronic infection of mice with Toxoplasma gondii. J Immunol 164: 4826–4834, 2000
Matyszak MK, Perry VH: The potential role of dendritic cells in immune-mediated inflammatory diseases in the central nervous system. Neuroscience 74: 599–608, 1996
Carson MJ, Reilly CR, Sutcliffe JG, Lo D: Disproportionate recruitment of CD8+ T cells into the central nervous system by professional antigen-presenting cells. Am J Pathol 154: 481–494, 1999
Owens T, Renno T, Taupin V, Krakowski M: Inflammatory cytokines in the brain: does the CNS shape immune responses? Immunol Today 15: 566–571, 1994
Sampson JH, Archer GE, Ashley DM, Fuchs HE, Hale LP, Dranoff G, Bigner DD: Subcutaneous vaccination with irradiated, cytokine-producing tumor cells stimulates CD8+ cell-mediated immunity against tumors located in the ‘immunologically privileged’ central nervous system. Proc Natl Acad Sci USA 93: 10399–10404, 1996
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fecci, P.E., Mitchell, D.A., Archer, G.E. et al. The History, Evolution, and Clinical use of Dendritic Cell-Based Immunization Strategies in the Therapy of Brain Tumors. J Neurooncol 64, 161–176 (2003). https://doi.org/10.1023/A:1024943506506
Issue Date:
DOI: https://doi.org/10.1023/A:1024943506506