Abstract
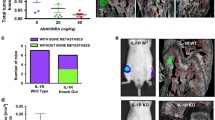
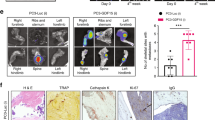
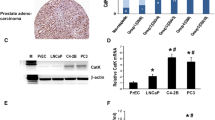
The growth of metastatic prostate cancer cells in the bone involves an intimate interaction between the tumor cells and various elements of the bone microenvironment, resulting in increased rate of bone turnover and rapid tumor growth. The αvβ3 integrin has been shown to play an important role in tumor growth and angiogenesis, and is known to be critical to osteoclast formation and activity. This study was designed to examine the role of αvβ3 expressed by cells native to the bone in the growth and pathogenesis of prostate cancer bone metastases. Human prostate cancer cells which do not express αvβ3 or αIIbβ3 integrins were injected directly into human bone fragments previously implanted subcutaneously in SCID mice (SCID-human-bone model). At the same time treatment with anti-β3 antibody fragment (m7E3 F(ab′)2) i.p. at 300 μg/dose 3× per week was initiated and continued for 2 weeks. In this system, m7E3 F(ab′)2 only recognizes human bone-derived αvβ3. Antibody inhibition of αvβ3 integrin in vivo resulted in a specific reduction in the proportion of antigenically-human blood vessels within tumor-bearing bone implants (from 73.5% ± 3.93 in controls to 17.74% ± 5.64 in treated animals). Proliferation of the αvβ3-negative tumor cells was also reduced, although the overall vessel density was maintained by compensating mouse vasculature. Blockage of human bone-derived αvβ3 also significantly reduced the recruitment of osteoclasts in response to tumor cells, as well as degradation of calcified bone tissue. Together these observations confirm the importance of αvβ3 in bone metabolism and angiogenesis, and point to the role of these processes in controlling growth of metastatic prostate cancer cells in the bone.
Similar content being viewed by others
References
Gleave M, Hsieh JT, Gao CA et al. Acceleration of human prostate cancer growth in vivo by factors produced by prostate and bone fibroblasts. Cancer Res 1991; 51: 3753–61.
Chackal—Roy M, Niemeyer C, Moore M et al. Stimulation of human prostatic carcinoma cell growth by factors present in human bone marrow. J Clin Invest 1989; 84: 43–50.
Lang SH, Miller WR, Habib FK. Stimulation of human prostate cancer cell lines by factors present in human osteoblast—like cells but not in bone marrow. Prostate 1995; 27: 287–93.
Nemeth JA, Roberts JW, Mullins CM et al. Persistence of human vascular endothelium in experimental human prostate cancer bone tumors. Clin Exp Metastasis 2000; 18: 231–7.
Nemeth JA, Harb JF, Barroso Jr. U et al. Severe combined immunodeficient—hu model of human prostate cancer metastasis to human bone. Cancer Res 1999; 59: 1987–93.
Nemeth JA, Yousif R, Herzog M, Che M et al. Matrix metalloproteinase activity, bone matrix turnover, and tumor cell proliferation in prostate cancer bone metastasis. J Natl Cancer Inst 2002; 94: 17–25.
Thalmann GN, Sikes RA, Wu TT et al. LNCaP progression model of human prostate cancer: androgen—independence and osseous metastasis. Prostate 2000; 44: 91–103.
Wu TT, Sikes RA, Cui Q et al. Establishing human prostate cancer cell xenografts in bone: Induction of osteoblastic reaction by prostate—specific antigen—producing tumors in athymic and SCID/bg mice using LNCaP and lineage—derived metastatic sublines. Int J Cancer 1998; 77: 887–94.
Hynes RO. Integrins: Versatility, modulation, and signaling in cell adhesion. Cell 1992; 69: 11–25.
Varner JA, Cheresh DA. Integrins and cancer. Curr Opin Cell Biol 1996; 8: 724–30.
Knox JD, Cress AE, Clark V et al. Differential expression of extracellular matrix molecules and the alpha 6—integrins in the normal and neoplastic prostate. Am J Pathol 1994; 145: 167–74.
Nagle RB, Knox JD, Wolf C et al. Adhesion molecules, extracellular matrix, and proteases in prostate carcinoma. J Cell Biochem Suppl 1994; 19: 232–7.
Nagle RB, Hao J, Knox JD et al. Expression of hemidesmosomal and extracellular matrix proteins by normal and malignant human prostate tissue. Am J Pathol 1995; 146: 1498–507.
Bonkhoff H, Stein U, Remberger K. Differential expression of alpha 6 and alpha 2 very late antigen integrins in the normal, hyperplastic, and neoplastic prostate: Simultaneous demonstration of cell surface receptors and their extracellular ligands. Hum Pathol 1993; 24: 243–8.
Seftor RE, Seftor EA, Gehlsen KR et al. Role of the alpha v beta 3 integrin in human melanoma cell invasion. Proc Natl Acad Sci USA 1992; 89: 1557–61.
Felding—Habermann B, Cheresh DA. Vitronectin and its receptors. Curr Opin Cell Biol 1993; 5: 864–8.
Woodard AS, Garcia—Cardena G, Leong M et al. The synergistic activity of alphavbeta3 integrin and PDGF receptor increases cell migration. J Cell Sci 1998; 111: 469–78.
Zheng DQ, Woodard AS, Fornaro M et al. Prostatic carcinoma cell migration via alpha(v)beta3 integrin is modulated by a focal adhesion kinase pathway. Cancer Res 1999; 59: 1655–64.
Edlund M, Miyamoto T, Sikes RA et al. Integrin expression and usage by prostate cancer cell lines on laminin substrata. Cell Growth Differ 2001; 12: 99–107.
Griffioen AW, Molema G. Angiogenesis: Potentials for pharmacologic intervention in the treatment of cancer, cardiovascular diseases, and chronic inflammation. Pharmacol Rev 2000; 52: 237–68.
Eliceiri BP, Cheresh DA. The role of alphav integrins during angiogenesis: insights into potential mechanisms of action and clinical development. J Clin Invest 1999; 103: 1227–30.
Friedlander M, Brooks PC, Shaffer RW et al. Definition of two angiogenic pathways by distinct alpha v integrins.Science 1995; 270: 1500–2.
Thompson WD, Li WW, Maragoudakis M. The clinical manipulation of angiogenesis: Pathology, side—effects, surprises, and opportunities with novel human therapies. J Pathol 2000; 190: 330–7.
Brooks PC, Montgomery AM, Rosenfeld M et al. Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell 1994; 79: 1157–64.
Varner JA, Nakada MT, Jordan RE et al. Inhibition of angiogenesis and tumor growth by murine 7E3, the parent antibody of c7E3 Fab (abciximab; ReoPro). Angiogenesis 1999; 3: 53–60.
Davies J, Warwick J, Totty N, et al. The osteoclast functional antigen, implicated in the regulation of bone resorption, is biochemically related to the vitronectin receptor. J Cell Biol 1989; 109: 1817–26.
Horton MA, Davies J. Perspectives: Adhesion receptors in bone. J Bone Miner Res 1989; 4: 803–8.
Hughes DE, Salter DM, Dedhar S et al. Integrin expression in human bone. J Bone MinerRes 1993; 8: 527–33.
Rodan SB, Rodan GA. Integrin function in osteoclasts. J Endocrinol 1997; 154 Suppl: S47–56.
Sato M, Sardana MK, Grasser WA et al. Echistatin is a potent inhibitor of bone resorption in culture. J Cell Biol 1990; 111: 1713–23.
Horton MA, Taylor ML, Arnett TR et al. Arg—Gly—Asp (RGD) peptides and the anti—vitronectin receptor antibody 23C6 inhibit dentine resorption and cell spreading by osteoclasts. Exp Cell Res 1991; 195: 368–75.
Carron CP, Meyer DM, Engleman VW et al. Peptidomimetic antagonists of alphavbeta3 inhibit bone resorption by inhibiting osteoclast bone resorptive activity, not osteoclast adhesion to bone.J Endocrinol 2000; 165: 587–98.
Carron CP, Meyer DM, Pegg JA et al. A peptidomimetic antagonist of the integrin alpha(v)beta3 inhibits Leydig cell tumor growth and the development of hypercalcemia of malignancy. Cancer Res 1998; 58: 1930–5.
Engleman VW, Nickols GA, Ross FP et al. A peptidomimetic antagonist of the alpha(v)beta3 integrin inhibits bone resorption in vitro and prevents osteoporosis in vivo. J Clin Invest 1997; 99: 2284–92.
Boissy P, Machuca I, Pfaff M et al. Aggregation of mononucleated precursors triggers cell surface expression of alphavbeta3 integrin, essential to formation of osteoclast—like multinucleated cells. J Cell Sci 1998; 111: 2563–74.
Trikha M, Zhou Z, Timar J et al. Multiple roles for platelet GPIIb/IIIa and αvβ3 integrins in tumor growth, angiogenesis and metastasis. Cancer Res 2002; 62: 2824–33.
Tam SH, Sassoli PM, Jordan RE et al. Abciximab (ReoPro, chimeric 7E3 Fab) demonstrates equivalent affinity and functional blockade of glycoprotein IIb/IIIa and alpha(v)beta3 integrins. Circulation 1998; 98: 1085–91.
Parums DV, Cordell JL, Micklem K et al. JC70: A new monoclonal antibody that detects vascular endothelium associated antigen on routinely processed tissue sections. J Clin Pathol 1990; 43: 752–7.
Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science 1994;264: 569–71.
Stromblad S, Becker JC, Yebra M et al. Suppression of p53 activity and p21WAF1/CIP1 expression by vascular cell integrin alphaVbeta3 during angiogenesis. J Clin Invest 1996; 98: 426–33.
Fidler IJ, Wilmanns C, Staroselsky A et al. Modulation of tumor cell response to chemotherapy by the organ environment. Cancer Metastasis Rev 1994; 13: 209–22.
Berruti A, Dogliotti L, Tucci M et al. Metabolic bone disease induced by prostate cancer: Rationale for the use of bisphosphonates. J Urol 2001; 166: 2023–31.
Coleman RE. Should bisphosphonates be the treatment of choice for metastatic bone disease? Semin Oncol 2001; 28: 35–41.
Brooks PC, Stromblad S, Klemke R et al. Antiintegrin alpha v beta 3 blocks human breast cancer growth and angiogenesis in human skin. J Clin Invest 1995; 96: 1815–22.
Chen YQ, Trikha M, Gao X et al. Ectopic expression of platelet integrin alphaIIb beta3 in tumor cells from various species and histological origin. Int J Cancer 1997; 72: 642–8.
Trikha M, Timar J, Lundy SK et al. Human prostate carcinoma cells express functional alphaIIb(beta)3 integrin. Cancer Res 1996; 56: 5071–8.
Ross FP, Chappel J, Alvarez JI et al. Interactions between the bone matrix proteins osteopontin and bone sialoprotein and the osteoclast integrin alpha v beta 3 potentiate bone resorption. J Biol Chem 1993; 268: 9901–7.
McHugh KP, Hodivala—Dilke K, Zheng MH et al. Mice lacking beta3 integrins are osteosclerotic because of dysfunctional osteoclasts. J Clin Invest 2000; 105: 433–40.
Udagawa N, Takahashi N, Akatsu T et al. Origin of osteoclasts: Mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow—derived stromal cells. Proc Natl Acad Sci USA 1990; 87: 7260–4.
Liapis H, Flath A, Kitazawa S. Integrin alpha V beta 3 expression by bone—residing breast cancer metastases. Diagn Mol Pathol 1996; 5: 127–35.
Mundy GR. Mechanisms of osteolytic bone destruction. Bone 1991; 12: S1–6.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nemeth, J.A., Cher, M.L., Zhou, Z. et al. Inhibition of αvβ3 integrin reduces angiogenesis, bone turnover, and tumor cell proliferation in experimental prostate cancer bone metastases. Clin Exp Metastasis 20, 413–420 (2003). https://doi.org/10.1023/A:1025461507027
Issue Date:
DOI: https://doi.org/10.1023/A:1025461507027