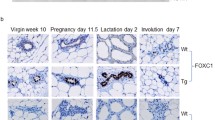
Abstract
The role of homeobox-containing genes in embryogenesis and organogenesis is well documented. Also, a sizeable body of evidence has accumulated and supports the fact that homeobox genes, when dysregulated, are involved in tumorigenesis. However, the precise mechanisms of homeobox gene functions are largely unknown. The mammary gland, in which most maturation occurs postnatally, provides an ideal model for studying the functions of homeobox genes in both development and tumorigenesis. The expression of many homeobox genes has been detected in both normal mammary gland and neoplastic breast tissues. In the normal mammary gland, the expression of homeobox genes is coordinately regulated by hormone and extracellular matrix (ECM) and other unknown factors in a spatial and temporal manner in both stromal and epithelial cells. Animals with misexpressed homeobox genes displayed different extents of defects in ductal proliferation, side branching, and alveoli formation, implying that homeobox genes are important for normal mammary gland development. Recent studies of homeobox genes in breast cancer cells and primary tumors indicate that they may also play a contributory or causal role in tumorigenesis by regulating the cell cycle, apoptosis, angiogenesis, and/or metastasis.
Similar content being viewed by others
REFERENCES
R. Krumlauf (1994). Hox genes in vertebrate development. Cell 70:191–201.
E. B. Lewis (1978). A gene complex controlling segmentation in Drosophila. Nature 276:565–570.
A. Chariot, J. Gielen, M. P. Merville, and V. Bours (1999). The homeodomain-containing proteins: An update on their interacting partners. Biochem. Pharmacol. 58:1851–1857.
R. S. Mann and M. Affolter (1998). Hox proteins meet more partners. Curr. Opin. Genet. Dev. 8:423–429.
R. Tupler, G. Perini, and M. R. Green (2001). Expressing the human genome. Nature 409:832–833.
S. Stein, R. Fritsch, L. Lemaire, and M. Kessel (1996). Checklist: Vertebrate homeobox genes. Mech. Dev. 55:91–108.
D. Duboule (1994). Guidebook to the Homeobox Genes. Oxford Press, Oxford University.
M. Maconochie, S. Nonchev, A. Morrison, and R. Krumlauf (1996). Paralogous hox genes: Function and regulation. Annu. Rev. Genet. 30:529–556.
D. Duboule and G. Morata (1994). Colinearity and functional hierarchy among genes of the homeotic complexes. Trends Genet. 10:358–364.
J. Van Oostveen, J. Bijl, F. Raaphorst, J. Walboomers, and C. Meijer (1999). The role of homeobox genes in normal hematopoiesis and hematological malignancies. Leukemia 13:1675–1690.
J. Aubin, M. Lemieux, M. Tremblay, J. Berard, and L. Jeannotte (1997). Early postnatal lethality in Hoxa-5 mutant mice is attributable to respiratory tract defects. Dev. Biol. 192:432–445.
A. Garcia-Gasca and D. D. Spyropoulos (2000). Differential mammary morphogenesis along the anteroposterior axis in Hox c6 gene targeted mice. Dev. Dyn. 219:261–276.
C. Cillo, A. Faiella, M. Cantile, and E. Boncinelli (1999). Homeobox genes and cancer. Exp. Cell. Res. 248:1–9.
C. Abate-Shen (2002). Deregulated homeobox gene expression in cancer: Cause or consequence? Nat. Rev. Cancer. 2:777–785.
H. J. Lawrence, G. Sauvageau, R. K. Humphries, and C. Largman (1996). The role of HOX homeobox genes in normal and leukemic hematopoiesis. Stem. Cells 14:281–291.
T. Kawabe, A. J. Muslin, and S. J. Korsmeyer (1997). HOX11 interacts with protein phosphatases PP2A and PP1 and disrupts a G2/M cell-cycle checkpoint. Nature 385:454–458.
M. Hatano, C. W. Roberts, M. Minden, W. M. Crist, and S. J. Korsmeyer (1991). Deregulation of a homeobox gene, HOX11, by the t(10;14) in T cell leukemia. Science 253:79–82.
C. C. Maulbecker and P. Gruss (1993). The oncogenic potential of deregulated homeobox genes. Cell. Growth. Diff. 4:431–441.
G. M. Edelman and F. S. Jones (1993). Outside and downstream of the homeobox. J. Biol. Chem. 268:20683–20686.
C. Cillo (1994–95). HOX genes in human cancers. Invasion Metastasis. 14:38–49.
M. T. Lewis (2000). Homeobox genes in mammary gland development and neoplasia. Breast Cancer Res. 2:158–169.
M. C. Neville, C. W. Daniel, (eds). The Mammary Gland Development, Regulation and Function. Plenum, New York, 1987.
Y. Friedmann, C. A. Daniel, P. Strickland, and C. W. Daniel (1994). Hox genes in normal and neoplastic mouse mammary gland. Cancer Res. 54:5981–5985.
X. Zhang, T. Zhu, Y. Chen, H. C. Mertani, K. O. Lee, and P. E. Lobie (2003). Human growth hormone-regulated HOXA1 is a human mammary epithelial oncogene. J. Biol. Chem. 278:7580–7590.
A. Srebrow, Y. Friedmann, A. Ravanpany, C. W. Daniel, and M. J. Bissel (1998). Expression of HoxA1 and HoxB7 is regulated by extracellular matrix-dependent signals in mammary epithelial cells. J. Cell Biochem. 69:377–391.
F. Chen and M. R. Capecchi (1999). Paralogous mouse Hox genes, Hoxa9, Hoxb9, and Hoxd9, function together to control development of the mammary gland in response to pregnancy. Dev. Biol. 96:541–546.
Y. Friedmann and C. W. Daniel (1996). Regulated expression of homeobox genes Msx-1 and Msx-2 in mouse mammary gland development suggests a role in hormone action and epithelialstromal interactions. Dev. Biol. 177:347–355.
D. J. Phippard, S. J. Weber-Hall, P. T. Sharpe, M. S. Naylor, H. Jayatalake, R. Maas, I. Woo, D. Roberts-Clark, P. H. Francis-West, Y. H. Liu, R. Maxson, R. E. Hill, and T. C. Dale (1996). Regulation of Msx-1, Msx-2, Bmp-2 and Bmp-4 during foetal and postnatal mammary gland development. Development 122:2729–2737.
B. Jehn, G. Chicaiza, F. Martin, and R. Jaggi (1994). Isolation of three novel pou-domain containing cDNA clones from lactating mouse mammary gland. Biochem. Biophys. Res. Commun. 200:156–162.
M. I. Morasso, K. A. Mahon, T. D. Sargent (1995). A Xenopus distal-less gene in transgenic mice: Conserved regulation in distal limb epidermis and other sites of epithelial-mesenchymal interaction. Proc. Natl. Acad. Sci. U. S. A. 92:3968–3972.
R. Hudson, A. Taniguchi-Sidle, K. Boras, O. Wiggan, P. A. Hamel (1998). Alx-4, a transcriptional activator whose expression is restricted to sites of epithelial-mesenchymal interactions. Dev. Dyn. 213:159–169.
A. Chariot, S. Senterre-Lesenfants, M. E. Sobel, and V. Castronovo (1998). Molecular cloning of a mutated HOXB7 cDNA encoding a truncated transactivating himeodomaincontaining protien. J. Cell Biochem. 71:46–54.
W. F. Odenwald, J. Garbern, H. Arnheiter, E. Tournier-Lasserve, and R. A. Lazzarini (1989). The Hox-1.3 homeo box protein is a sequence-specific DNA-binding phosphoprotein. Genes Dev. 3:158–172.
V. Raman, S. A. Martensen, D. Reisman, E. Evron, W. F. Odenwald, E. Jaffee, J. Marks, and S. Sukumar (2000). CompromisedHOXA5 function can limit p53 expression in human breast tumors. Nature 405:974–978.
M. T. Lewis, S. Ross, P. A. Strickland, C. J. Snyder, and C. W. Daniel (1999). Regulated expression patterns of IRX-2, an Iroquois-class homeobox gene, in the human breast. Cell Tissue Res. 296:549–554.
A. Krasner, L. Wallace, A. Thiagalingam, C. Jones, C. Lengauer, L. Minahan, Y. Ma, L. Kalikin, A. P. Feinberg, E. W. Jabs, A. Tunnacliffe, S. B. Baylin, D. W. Ball, and B. D. Nelkin (2000). Cloning and chromosomal localization of the human BARX2 homeobox protein gene. Gene 250:171–180.
C. Geserick, B. Weiss, W. D. Schleuning, B. Haendler (2002). OTEX, an androgen-regulated human member of the pairedlike class of homeobox genes. Biochem. J. 366:367–375.
G. B. Silberstein, G. R. Dressler, K. Van Horn (2002). Expression of the PAX2 oncogene in human breast cancer and its role in progesterone-dependent mammary growth. Oncogene 21:1009–1016.
J. C. Adams and F. M. Watt (1993). Regulation of development and differentiation by the extracellular matrix. Development 117:1183–1198.
M. Martins-Greenf and M. J. Bissell (1995). Cell-extracellular matrix interactions in development. Semin. Dev. 6:149–159.
W. Balesmans and W. Van Hul (2002). Extracellular regulation of BMP signaling in vertebrates: A cocktail of modulators. Dev. Biol. 250:231–250.
S. Vainio, I. Karavanova, A. Jowett, and I. Thesleff (1993). Identification of BMP-4 as a signal mediating secondary induction between epithelial and mesenchymal tissues during early tooth development. Cell 75:45–58.
N. R. Dunn, G. E. Winnier, L. K. Hargett, J. J. Schrick, A. B. Fogo, and B. L. Hogan (1997). Haploinsufficient phenotypes in Bmp4 heterozygous null mice and modification by mutations in Gli3 and Alx4. Dev. Biol. 188:235–247.
F. R. Goodman and P. J. Scambler (2001). Human HOX gene mutations. Clin. Genet. 59:1–11.
G. Hu, H. Lee, S. M. Price, M. M. Shen, and C. Abate-Shen (2001). Msx homeobox genes inhibit differentiation through upregulation of cyclin D1. Development 128:2373–2384.
I. Satokata, L. Ma, H. Ohshima, M. Bei, I. Woo, K. Nishizawa, T. Maeda, Y. Takano, M. Uchiyama, S. Heaney, H. Peters, Z. Tang, R. Maxson, and R. Maas (2000). Msx2 deficiency in mice causes pleiotropic defects in bone growth and ectodermal organ formation. Nat. Genet. 24:391–395.
I. Satokata and R. Maas (1994). Msx1 deficient mice exhibit cleft palate and abnormalities of craniofacial and tooth development. Nat. Genet. 6:348–356.
W. McGinnis and R. Krumlauff (1992). Homeobox genes and axial patterning. Cell 68:283–302.
F. F. Bolander (1990). Differential characteristics of the thoracic and abdominal mammary glands from mice. Exp. Cell. Res. 189:142–144.
T. Kamalati, B. Niranjan, J. Yant, and L. Buluwela (1999). HGF/SF in mammary epithelial growth and morphogenesis: In vitro and in vivo models. J. Mammary Gland Biol. Neoplasia 4:69–77.
J. P. Lydon, F. J. DeMayo, C. R. Funk, S. K. Mani, A. R. Hughes, C. A. Montgomery, G. ShyamalaJr., O. M. Conneely, and B. W. O'Malley (1995). Mice lacking progesterone receptor exihibit pleiotropic reproductive abnormalities. Genes Dev. 9:2266–2278.
V. Raman, A. Tamori, M. Vali, K. Zeller, D. Korz, and S. Sukumar (2000). HOXA5 regulates expression of the progesterone receptor. J. Biol. Chem. 275:26551–26555.
W. P. Bocchinfuso and K. S. Korach (1997). Mammary gland development and tumorigenesis in estrogen receptor knockout mice. J. Mammary Gland Biol. Neoplasia 2:323–334.
W. P. Bocchinfuso, J. K. Lindzey, S. C. Hewitt, J. A. Clark, P. H. Myers, R. Cooper, and K. S. Korach (2000). Induction of mammary gland development in estrogen receptor-alpha knockout mice. Endocrinology 141:2982–2994.
D. Duboule (2000). Developmental genetics. A Hox by any other name. Nature 403:609–610.
J. M. Greer, J. Puetz, K. R. Thomas, and M. R. Capecchi (2000). Maintenance of functional equivalence during paralogous Hox gene evolution. Nature 403:661–665.
J. Zakany, C. Fromental-Ramain, X. Warot, and D. Duboule (1997). Regulation of number and size of digits by posterior Hox genes: A dose-dependent mechanism with potential evolutionary implications. Proc. Natl. Acad. Sci. U. S. A. 94:13695–13700.
A. Chariot and V. Castronovo (1996). Detection ofHOXA1expression in human breast cancer. Biochem. Biophys. Res. Commun. 222:292–297.
A. Chariot, V. Castronovo, P. Le, C. Gillet, M. E. Sobel, and J. Gielen (1996). Cloning and expression of a new HOXC6 transcript encoding a repressing protein. Biochem. J. 319:91–97.
B. Bodey, B. Bodey, Jr, A. M. Groger, S. E. Siegel, and H. E. Kaiser (2000). Immunocytochemical detection of homeobox B3, B4, and C6 gene product expression in lung carcinomas. Anticancer. Res. 20:2711–2716.
H. L. Ford, E. N. Kabingu, E. A. Bump, G. L. Mutter, and A. B. Pardee (1998). Abrogation of the G2 cell cycle checkpoint associated with overexpression of HSIX1: A possible mechanism of breast carcinogenesis. Proc. Natl. Acad. Sci. U. S. A. 95:12608–12613.
T. Jin, D. R. Branch, X. Zhang, S. Qi, B. Youngson, and P. E. Goss (1999). Examination of POU homeobox gene expression in human breast cancer cells. Int. J. Cancer 81:104–112.
V. Budhram-Mahadeo, D. Ndisang, T. Ward, B. L. Weber, and D. S. Latchman (1999). The Brn-3b POU family transcription factor represses expression of the BRCA-1 anti-oncogene in breast cancer cells. Oncogene 18:6684–6691
J. H. Dennis, V. Budhram-Mahadeo, and D. S. Latchman (2001). The Brn-3b POU family transcription factor regulates the cellular growth, proliferation, and anchorage dependence of MCF7 human breast cancer cells. Oncogene 20:4961–4971.
B. Goulet, P. Watson, M. Poirier, L. Leduy, G. Berube, S. Meterissian, P. Jolicoeur, and A. Nepveu (2002). Characterization of a tissue-specific CDP/Cux isoform, p75, activated in breast tumor cells. Cancer Res. 62:6625–6633.
T. Jacks and R. A. Weinberg (1998). The expanding role of cell cycle regulators. Science 280:1035–1036.
H. L. Ford (1998). Homeobox genes: A link between development, cell cycle, and cancer? Cell Biol. Int. 22:397–400.
R. C. Smith, D. Branellec, D. H. Gorski, K. Guo, H. Perlman, J. F. Dedieu, C. Pastore, A. Mahfoudi, P. Denefle, J. M. Isner, and K. Walsh (1997). p21CIP1-mediated inhibition of cell proliferation by overexpression of the gax homeodomain gene. Genes Dev. 11:1674–1689.
M. R. Hough, M. D. Reis, R. Singaraja, D. M. Bryce, S. Kamel-Reid, I. Dardick, M. L. Breitman, I. D. Dube (1998). A model for spontaneous B-lineage lymphomas in IgHmu-HOX11 transgenic mice. Proc. Natl. Acad. Sci. U. S. A. 95:13853–13858.
N. Y. Rots, M. Liu, E. C. Anderson, and L. P. Freedman (1998). A differential screen for ligand-regulated genes: Identification of HoxA10 as a target of vitamin D3 induction in myeloid leukemic cells. Mol. Cell Biol. 18:1911–1918.
A. Nepveu (2001). Role of the multifunctional CDP/Cut/Cux homeodomain transcription factor in regulating differentiation, cell growth and development. Gene 270:1–15.
T. C. Wang, R. D. Cardiff, L. Zukerberg, E. Lees, A. Arnold, and E. V. Schmidt (1994). Mammary hyperplasia and carcinoma in MMTV-cyclin D1 transgenic mice. Nature 369:669–671.
S. Boulon, J. C. Dantonel, V. Binet, A. Vie, J. M. Blanchard, R. A. Hipskind, and A. Philips (2002). Oct-1 potentiates CREBdriven cyclin D1 promoter activation via a phospho-CREBand CREB binding protein-independent mechanism. Mol. Cell Biol. 22:7769–7779.
X. F. Qin, Y. Luo, H. Suh, J. Wayne, Z. Misulovin, R. G. Roeder, and M. C. Nussenzweig (1994). Transformation by homeobox genes can be mediated by selective transcriptional repression. EMBO J. 13:5967–5976.
W. Risau (1997). Mechanisms of angiogenesis. Nature 386:671–674.
D. Hanahan and J. Folkman (1996). Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 86:353–364.
P. Carmeliet and R. K. Jain (2000). Angiogenesis in cancer and other diseases. Nature 407:249–257.
A. Care, A. Silvani, E. Meccia, G. Mattia, A. Stoppacciaro, G. Parmiani, C. Peschle, and M. P. Colombo (1996). HOXB7 constitutively activates basic fibroblast growth factor in melanomas. Mol. Cell Biol. 16:4842–4851.
A. Care, F. Felicetti, E. Meccia, L. Bottero, M. Parenza, A. Stoppacciaro, C. Peschle, and M. P. Colomb (2001). HOXB7: A key factor for tumor-associated angiogenic switch. Cancer Res. 61:6532–6539.
A. Carè, A. Silvani, E. Meccia, G. Mattia, C. Peschle, M. P. Colombo (1998). Transduction of the SkBr3 breast carcinoma cell line with the HOXB7 gene induces bFGF expression, increases cell proliferation and reduces growth factor dependence. Oncogene 16:3285–3289.
A. J. Hayes, W. Q. Huang, J. Yu, P. C. Maisonpierre, A. Liu, F. G. Kern, M. E. Lippman, S. W. McLeskey, and L. Y. Li (2000). Expression and function of angiopoietin-1 in breast cancer. Br. J. Cancer 83:1154–1160.
V. Budhram-Mahadeo, M. Parker, and D. S. Latchman (1998). POU transcription factors Brn-3a and Brn-3b interact with the estrogen receptor and differentially regulate transcriptional activity via an estrogen response element. Mol. Cell Biol. 18:1029–1041
J. F. Couse and K. S. Korach (1999). Estrogen receptor null mice: What havewelearned and where will they lead us? Endocr. Rev. 20:358–417.
G. I. Evan and K. H. Vousden (2001). Proliferation, cell cycle and apoptosis in cancer. Nature 411:342–348.
M. T. Debies and D. R. Welch (2001). Genetic basis of human breast cancer metastasis. J. Mammary Gland Biol. Neoplasia 6:441–451.
D. M. Loeb and S. Sukumar (2002). The role of WT1 in oncogenesis: Tumor suppressor or oncogene? Int. J. Hematol. 76:117–126.
M. Akam (1998). Hox genes: From master genes to micromanagers. Curr. Biol. 8:R676-R678.
A. Chariot, C. van Lint, M. Chapelier, J. Gielen, M. P. Merville, and V. Bours (1999). CBP and histone deacetylase inhibition enhance the transactivation potential of the HOXB7 homeodomain-containing protein. Oncogene 18:4007–1014.
J. B. Gibbs (2000). Mechanism-based target identification and drug discovery in cancer research. Science 287:1969–1973.
M. Windschwendter and P. A. Jones (2002). DNA methylation and breast carcinogenesis. Onocogene 21:5462–5482.
G. R. Cunha, P. Young, Y. K. Hom, P. S. Cooke, J. A. Taylor, and D. B. Lubahn (1997). Elucidation of a role for stromal steroid hormone receptors in mammary gland growth and development using tissue recombinants. J. Mammary Gland Biol. Neoplasia 2:393–402.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, H., Sukumar, S. Role of Homeobox Genes in Normal Mammary Gland Development and Breast Tumorigenesis. J Mammary Gland Biol Neoplasia 8, 159–175 (2003). https://doi.org/10.1023/A:1025996707117
Issue Date:
DOI: https://doi.org/10.1023/A:1025996707117