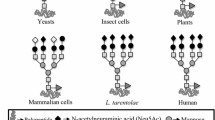
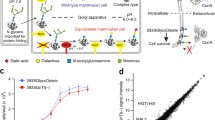
Abstract
The analysis of many natural glycoproteins and their recombinant counterparts from mammalian hosts has revealed that the basic oligosaccharide structures and the site occupancy of glycosylated polypeptides are primarily dictated by the protein conformation.
The equipment of many frequently used host cells (e.g. BHK-21 and CHO-cells) with glycosyltransferases, nucleotide-sugar synthases and transporters appears to be sufficient to guarantee complex-type glycosylation of recombinant proteins with a high degree of terminal α2-3 sialylation even under high expression conditions. Some human tissue-specific terminal carbohydrate motifs are not synthesized by these cells since they lack the proper sugar-transferring enzymes (e.g. α1-3/4 fucosyltransferases, α2-6 sialyltransferases). Glycosylation engineering of these hosts by stable transfection with genes encoding terminal human glycosyltransferases allows to obtain products with tailored (human tissue-specific) glycosylation in high yields.
Using site-directed mutagenesis, unglycosylated polypeptides can be successfully converted in N- and/or O-glycoproteins by transferring glycosylation domains (consisting of 7-17 amino acids) from donor glycoproteins to different loop regions of acceptor proteins.
The genetic engineering of glycoproteins and of host cell lines are considered to provide a versatile tool to obtain therapeutic glyco-products with novel/improved in-vivo properties, e.g. by introduction of specific tissue-targeting signals by a rational design of terminal glycosylation motifs.
Similar content being viewed by others
References
Varki A (1993) Glycobiology 3: 97–130.
Berman PW, Lasky LA (1985) Trends Biochem Tech 3: 51–3.
Kobata A (1992) Eur J Biochem 209: 483–501.
Schachter H (1994) In Molecular Glycobiology (Fukuda M, Hindsgaul O, eds.), pp 88–162. Oxford, UK: Oxford University Press.
Hoffmann A, Nimtz M, Wurster U, Conradt HS (1994) J Neurochem 63: 2185–196.
Hoffmann A, Nimtz M, Getzlaff R, Conradt HS (1995) FEBS Lett 359: 164–68.
Pohl S, Hoffmann A, Rüdiger A, Nimtz M, Jaeken J, Conradt HS (1997) Glycobiology 7: 1077–84.
Conradt HS, Lindenmaier W, Ausmeier M, Hauser H, Dittmar KEJ (1986) Carbohydr Res 49: 443–50.
Conradt HS, Egge H, Peter-Katalinic J, Reiser W, Siklosi T, Schaper K (1987) J Biol Chem 262: 14600–605.
Mutsaers JHGM, Kamerling JP, Devos R, Guisez Y, Fiers W, Vliegenthart JFG (1986) Eur J Biochem 156: 651–54.
Conradt HS, Hofer B, Hauser H (1990) Trends Glycosci Glycotechnol 2: 168–81.
Goochee CF, Monica T (1990) Bio/Technology 8: 421–27.
Goochee CF, Gramer MJ, Andersen DC, Bahr JB, Rasmussen JR (1991) Bio/Technology 9: 1347–55.
Jenkins N, Parekh RB, James DC (1996) Nature Biotechnol 14: 975–81.
Grabenhorst E, Nimtz M, Conradt HS, unpublished results.
Grabenhorst E, Hoffmann A, Nimtz M, Zettlmeiµ G, Conradt HS (1995) Eur J Biochem 232: 718–25.
Schlenke P, Grabenhorst E, Nimtz M, Conradt HS (1998) Cytotechnology, in press.
Grabenhorst E, Hofer B, Nimtz M, Jäger V, Conradt HS (1993) Eur J Biochem 215: 189–97.
Ackermann M (1995) Doctoral thesis, Technical University of Braunschweig,Germany.
Ackermann M, Nimtz M, Grabenhorst E, Conradt HS, Jäger V (1995) In The Baculovirus and Insect Cell Gene Expression Conference, Pinehurst, NC, USA (abstract).
Knepper TP, Arbogast B, Schreurs J, Deinzer ML (1992) Biochemistry 31: 11651–59.
James DC, Freedman RB, Hoare M, Ogonah OW, Rooney BC, Larionov OA, Dobrovolsky VN, Lagutin OV, Jenkins N (1995) Bio/Technology 13: 592–96.
Grabenhorst E (1994) Doctoral thesis, Technical University of Braunschweig,Germany.
Dittmar KEJ, Conradt HS, Hauser H, Hofer B, Lindenmaier W (1989) In Advances in Protein Design (Blöcker H, Collins J, Schmidt RD, Schomburg D, eds.), GBF-Monographs vol. 12, pp145–56. Weinheim, New York: VCH Publishers.
Kagawa Y, Takasaki S, Utsumi J, Hosoi K, Shimizu H, Kochibe N, Kobata A (1988) J Biol Chem 263: 17508–15.
Zettlmeissl G, Conradt HS, Nimtz M, Karges P (1989) J Biol Chem 264: 21153–59.
Sasaki H, Bothner B, Dell A, Fukuda M (1987) J Biol Chem 262: 12059–76.
Takeuchi M, Takasaki S, Miyazaka H, Kato T, Hoshi S, Kochibe N, Kobata A (1988) J Biol Chem 263: 3657–63.
Nimtz M, Wray V, Augustin M, Klöppel K-D, Conradt HS (1993) Eur J Biochem 213: 39–56.
Hokke CH, Bergwerff AA, van Dedem GWK, Kamerling JP, Vliegenthart JFG (1995) Eur J Biochem 228: 981–1008.
Grabenhorst E (1991) Diploma thesis, Technical University of Braunschweig,Germany.
Conradt HS, Gawlitzek M, Grabenhorst E, Hoffmann A, Nimtz M, Oltmanns-Bleck F, Pohl S (1997) In Medicinal Chemistry: Today andTomorrow (Yamazaki M,ed) pp 205–12. Blackwell Science.
Conradt HS, Nimtz M, Dittmar KEJ, Lindenmaier W, Hoppe J, Hauser H (1989) J Biol Chem 264: 17368–73.
Costa J, Grabenhorst E, Nimtz M, Conradt HS (1997) J Biol Chem 272: 11613–21.
Grabenhorst E, Nimtz M, Costa J, Conradt HS (1998) J Biol Chem, in press.
Kukowska-Latallo JF, Larsen RD, Nair RP, Lowe JB (1990) Genes Dev 4: 1288–1303.
Goelz S, Hession C, Goff D, Griffiths B, Tizard R, Newman B, Chi-Rosso G, Lobb R (1990) Cell 63: 1349–56.
Weston BW, Nair RP, Larsen RD, Lowe JB (1992) J Biol Chem 267: 4152–60.
Koszdin KL, Bowen BR (1992) Biochem Biophys Res Commun 187: 152–57.
Weston BW, Smith PL, Kelly RJ, Lowe JB (1992) J Biol Chem 267: 24575–84.
Sasaki K, Kurata K, Funayama K, Nagata M, Watanabe E, Ohta S, Hanai N, Nishi T (1994) J Biol Chem 269: 14730–37.
Varki A (1994) Proc Natl Acad Sci USA 91: 7390–97.
Lasky LA (1995) Ann Rev Biochem 64: 113–39.
McEver RP, Moore KL, Cummings RD (1995) J Biol Chem 270: 11025–28.
Kansas GS (1996) Blood 88: 3259–87.
de Graaf TW, van der Stelt ME, Anbergen MG, van Dijk W (1993) J Exp Med 177: 657–66.
Brinkman-van der Linden EC, de Haan PF, Havenaar EC, van Dijk W (1998) Glycoconj J 15: 177–82.
de Vries T, Srnka CA, Palcic MM, Swiedler SJ, van den Eijnden DH, Macher BA (1995) J Biol Chem 270: 8712–22.
de Vries T, Palcic MP, Schoenmakers PS, van den Eijnden DH, Joziasse DH (1997) Glycobiology 7: 921–27.
Shinoda K, Morishita Y, Sasaki K, Matsuda Y, Takahashi I, Nishi T (1997) J Biol Chem 272: 31992–97.
Britten CJ, van den Eijnden DH, McDowell W, Kelly VA, Witham SJ, Edbrooke MR, Bird MI, de Vries T, Smithers N (1998) Glycobiology 8: 321–27.
Natsuka S, Gersten KM, Zenita K, Kannagi R, Lowe JB (1994) J Biol Chem 269: 16789–94.
Kumar R, Potvin B, Muller WA, Stanley P (1991) J Biol Chem 266: 21777–83.
Nimtz M, Grabenhorst E, Gambert U, Costa J, Wray V, Morr M, Thiem J, Conradt HS (1998) Glycoconj J 15: 873–83.
Hoffmann A, Nimtz M, Conradt HS (1997) Glycobiology 7: 499–506.
Smith DF, Larsen RD, Mattox S, Lowe JB, Cummings RD (1990) J Biol Chem 265: 6225–34.
Sueyoshi S, Tsuboi S, Sawada-Hirai R, Dang UN, Lowe JB, Fukuda M (1994) J Biol Chem 269: 32342–50.
Lowe JB, Kukowska-Latallo JF, Nair RP, Larsen RD, Marks RM, Macher BA, Kelly RJ, Ernst LK (1991) J Biol Chem 266: 17467–77.
Gersten K, Natsuka S, Trinchera M, Petryniak B, Kelly RJ, Hiraiwa N, Jenkins NA, Gilbert DJ, Copeland NG, Lowe JB (1995) J Biol Chem 270: 25047–56.
Weinstein J, Lee EU, McEntee K, Lai P-H, Paulson JC (1987) J Biol Chem 262: 17735–43.
Masri KA, Appert HE, Fukuda MN (1988) Biochem Biophys Res Commun 157: 657–63.
Larsen RD, Rajan VP, Ruff MM, Kukowska-Latallo J, Cummings RD, Lowe JB (1989) Proc Natl Acad Sci USA 86: 8227–31.
Homa FL, Hollander T, Lehman DJ, Thomsen DR, Elhammer AP (1993) J Biol Chem 268: 12609–16.
Jaskiewicz E, Guofen Zhu, Bassi R, Darling DS, Young WW Jr (1996) J Biol Chem 271: 26395–403.
Borsig L, Katopodis AG, Bowen BR, Berger EG (1998) Glycobiology 8: 259–68.
Kaplan HA, Woloski BMRNJ, Hellman M, Jamieson JC (1983) J Biol Chem 258: 11505–9.
Uejima T, Uemura M, Nozawa S, Narimatsu H (1992) Cancer Res 52: 6158–63.
Cho SK, Cummings RD (1997) J Biol Chem 272: 13622–28.
Zhu G, Allende ML, Jaskiewicz E, Qian R, Darling DS, Worth CA, Colley KJ, Young WW Jr (1998) Glycobiology 8: 831–40.
Grundmann U, Nerlich C, Rein T, Zettlmeissl G (1990) Nucl Acids Res 18: 667.
Joziasse DH, Schiphorst WECM, van den Eijnden DH, Van Kuik JA, Van Halbeek H, Vliegenthart JFG (1987) J Biol Chem 262: 2025–33.
Nemansky M, van den Eijnden DH (1992) Biochem J 287: 311–16.
Hokke CH, van der Ven JGM, Kamerling JP, Vliegenthart JFG (1993) Glycoconj J 10: 82–90.
Weisshaar G, Hiyama J, Renwick AGC, Nimtz M (1991) Eur J Biochem 195: 257–68.
Yan SB, Chao YB, van Halbeek H (1993) Glycobiology 3: 597–608.
Bergwerff AA, Van Oostrum J, Kamerling JP, Vliegenthart JFG (1995) Eur J Biochem 228: 113–26.
Colley KJ (1997) Glycobiology 7: 1–13.
Munro S (1998) Trends Cell Biol 8: 11–15.
Lowe M, Nakamura N, Warren G (1998) Trends Cell Biol. 8: 40–44.
Nakayama J, Fukuda MN, Fredette B, Ranscht B, Fukuda M (1995) Proc Natl Acad Sci USA 92: 7031–35.
Eckhardt M, Mühlenhoff M, Bethe A, Koopman J, Frosch M, Gerardy-Schahn R (1995) Nature 373: 715–18.
Bretscher MS, Munro S (1993) Science 261: 1280–81.
Grabenhorst E, Nimtz M, Conradt HS, manuscript in preparation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grabenhorst, E., Schlenke, P., Pohl, S. et al. Genetic engineering of recombinant glycoproteins and glycosylation pathway in mammalian host cells. Glycoconj J 16, 81–97 (1999). https://doi.org/10.1023/A:1026466408042
Issue Date:
DOI: https://doi.org/10.1023/A:1026466408042