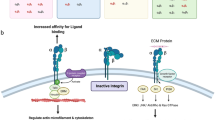
Abstract
Epithelial cancer of the ovary spreads by implantation of tumor cells onto the mesothelial cells that line the peritoneal cavity. The aim of this study was to identify the cell–matrix interactions that mediate ovarian carcinoma cell migration toward components of the mesothelial cell-associated extracellular matrix. The human ovarian carcinoma cell lines NIH:OVCAR5 and SKOV3 were analyzed by flow cytometry for the expression of cell surface receptors. The ability of those receptors to mediate ovarian carcinoma cell migration toward fibronectin, type IV collagen, and laminin was determined. A monoclonal antibody against the β1 integrin subunit abrogated the migration of both cell lines toward the extracellular matrix proteins. Blocking antibodies against alpha integrin subunits suggest that ovarian carcinoma cell migration toward fibronectin is primarily mediated by the ∝5β1 integrin, type IV collagen by the ∝2β1 integrin, and laminin by the ∝6β1 integrin. These results suggest that ovarian carcinoma cell migration is regulated by multiple β1 integrin–matrix interactions. Significant reduction of cell migration was observed with a monoclonal antibody against CD44 that blocks the hyaluronan-binding site of CD44, but not with an antibody that binds at an alternate site on CD44. Intact hyaluronan and/or hyaluronan oligomers also inhibited cell migration, suggesting that the CD44–hyaluronan interaction provides an integrin-independent mechanism of control for ovarian carcinoma cell migration. These results suggest that ovarian carcinoma cell migration is regulated by both integrin-dependent mechanisms, involving the interaction of β1 integrins with extracellular matrix proteins, and an integrin-independent mechanism that involves the interaction of CD44 and hyaluronan.
Similar content being viewed by others
References
Daly M, Obrams GI. Epidemiology and risk assessment for ovarian cancer. Semin Oncol 1998; 25: 255–64.
Neidbala MJ, Crickard K, Bernacki RJ. Interactions of human ovarian tumor cells with human mesothelial cells grown on extracellular matrix. Exp Cell Res 1985; 160: 499–513.
Lessan K, Aguiar DJ, Oegema T et al. CD44 and β1 integrin mediate ovarian carcinoma cell adhesion to peritoneal mesothelial cells. Am J Pathol 1999; 154: 1525–37.
Cannistra SA, Kansas GF, Niloff J et al. Binding of ovarian cancer cells to peritoneal mesothelium in vitro is partly mediated by CD44H. Cancer Res 1993; 52: 3830–8.
Jonjic N, Peri G, Bernascorri S, et al. Expression of adhesion molecules and chemotactic cytokines in cultured human mesothelial cells. J Exp Med 1992; 176: 1165–74.
Rieppi M, Vergani V, Gatto C et al. Mesothelial cells induce the motility of human ovarian carcinoma cells. Int J Cancer 1999; 80: 303–7.
Hynes RO. Integrins: Versatility, modulation, and signaling in cell adhesion. Cell 1992; 69: 11–25.
Tozer EC, Hughes PE, Loftus JC. Ligand binding and affinity modulation of integrins. Biochem Cell Biol 1996; 74: 785–98.
Boudreau N, Bissell MJ. Extracellular matrix signalling: Integration of form and function in normal and malignant cells. Curr Opin Cell Biol 1998; 10: 640–6.
Sheppard D. Epithelial integrins. BioEssays 1996; 18: 655–60.
Aruffo A, Stamenkovic I, Melnick M et al. CD44 is the principal cell surface receptor for hyaluronate. Cell 1990; 61: 1303–13.
Jalkanen S, Jalkanen M. Lymphocyte CD44 binds the COOH-terminal heparin-binding domain of fibronectin. J Cell Biol 1992; 116: 817–25.
Jones LM, Gardner MJ Catterall JB, Turner GA. Hyaluronic acid secreted by mesothelial cells: A natural barrier to ovarian cancer cell adhesion. Clin Exp Metastasis 1995; 13: 373–80.
Gardner MJ, Catterall JB, Jones LM, Turner GA. Human ovarian tumour cells can bind hyaluronic acid via membrane CD44: A possible step in peritoneal metastasis. Clin Exp Metastasis 1996; 14: 325–34.
Ladeda V, Aguirre Ghiso JA, Bal de Kier Joffe E. Function and expression of CD44 during spreading, migration, and invasion of murine carcinoma cells. Exp Cell Res 1998; 242: 515–27.
Strobel T, Swanson L, Cannistra SA. In vivo inhibition of CD44 limits intra-abdominal spread of a human ovarian cancer xenograft in nude mice: A novel role for CD44 in the process of peritoneal implantation. Cancer Res 1997; 57: 1228–32.
Zeng C, Toole BP, Kinney SD et al. Inhibition of tumor growth in vivo by hyaluronan oligomers. Int J Cancer 1998; 77: 396–401.
Lesley J, Hyman R, English N et al. CD44 in inflammation and metastasis. Glyco J 1997; 14: 611–22.
Salmi M, Gron-Virta K, Sointu P et al. Regulated expression of exon 6-containing isoforms of CD44 in man: Downregulation during malignant transformation of tumors of squamocellular origin. J Cell Biol 1993; 122: 431–42.
Cannistra SA, Abu-Jawdeh G, Niloff J et al. CD44 variant expression is a common feature of epithelial ovarian cancer: Lack of association with standard prognostic factors. J Clin Oncol 1995; 13: 1912–21.
Molpus KL, Koelliker D, Atkins L et al. Characterization of a xenograft model of human ovarian carcinoma which produces intraperitoneal carcinomatosis and metastases in mice. Int J Cancer 1996; 68: 588–95.
Trempe G, Fogh J. New human tumor cell lines. In Fogh J (ed): Human Tumor Cells in Vitro. New York: Plenum Press 1975; 115–59.
Hamilton TC, Young RC, Ozols RF. Experimental model systems of ovarian cancer: Applications to the design and evaluation of new treatment approaches. Semin Oncol 1984; 11: 285–98.
McCarthy JB, Skubitz APN, Palm SL, Furcht LT. Metastasis inhibition of different tumor types by purified laminin fragments and a heparin-binding fragment of fibronectin. J Natl Cancer Inst 1988; 80: 108–16.
Smith DE, Mosher DF, Johnson RB. Immunological identification of two sulfhydryl-containing fragments of human plasma fibronectin. J Biol Chem 1982; 257: 5831–8.
Knudson W, Aguiar DJ, Hua Q, Knudson CB. CD44-anchored hyaluronan-rich pericellular matrices. Exp Cell Res 1996; 228: 216–28.
Pattaramalai S, Skubitz KM, Skubitz APN. A novel recognition site on laminin for the α3β1 integrin. Exp Cell Res 1996; 222: 281–90.
Yen CJ, Fang CC, Chen YM, et al. Extracellular matrix proteins modulate human peritoneal mesothelial cell behavior. Nephron 1997; 75: 188–95.
Perfumo F, Altieri P, Degl’Innocenti ML et al. Effects of peritoneal effluents on mesothelial cells in culture: Cell proliferation and extracellular matrix regulation. Nephrol Dial Transplant 1996; 11: 1803–9.
Radford KJ, Thorne RF, Hersey P. Regulation of tumor cell motility and migration by CD63 in a human melanoma cell line, J Immunol 1997; 158: 3353–8.
Masser K, Wolf K, Klein CE et al. Functional heirarchy of simultaneously expressed adhesion receptors: Integrin α2β1 but not CD44 mediates MV3 melanoma cell migration and matrix reorganization within three-dimensional hyaluronan-containing collagen matrices. Mol Biol Cell 1999; 10: 3067–79.
Gardner MJ, Lindsay MH, Catterall JB et al. Expression of cell adhesion molecules on ovarian tumor cell lines and mesothelial cells, in relation to ovarian cancer metastasis. Cancer Lett 1995; 91: 229–34.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Casey, R.C., Skubitz, A.P. CD44 and β1 integrins mediate ovarian carcinoma cell migration toward extracellular matrix proteins. Clin Exp Metastasis 18, 67–75 (2000). https://doi.org/10.1023/A:1026519016213
Issue Date:
DOI: https://doi.org/10.1023/A:1026519016213