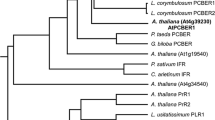
Abstract
Quinolate acid phosphoribosyltransferase (QPRTase), a key enzyme in nicotinamide adenine dinucleotide (NAD) biosynthesis, also plays an important role in ensuring nicotinic acid is available for the synthesis of defensive pyridine alkaloids in Nicotiana species. In this study, cDNAs for QPRTase were characterized from N. rustica and N. tabacum. Deduced proteins from both cDNAs are almost identical and contain a 24 amino acid N-terminal extension, not reported in other QPRTases, that has characteristics of a mitochondrial targeting sequence. In N. tabacum and N. sylvestris, both of which contain nicotine as the major pyridine alkaloid, QPRTase transcript was detected in roots, the site of nicotine synthesis, but not in leaves. QPRTase transcript levels increased markedly in roots of both species 12–24 h after damage to aerial tissues, with a concomitant rise in transcript levels of putrescine N-methyltransferase (PMT), another key enzyme in nicotine biosynthesis. In N. glauca, however, in which anabasine represents the major pyridine alkaloid, QPRTase transcript was detected in both leaf and root tissues. Moreover, wound induction of QPRTase but not PMT was observed in leaf tissues, and not in roots, 12–24 h after wounding. Southern analysis of genomic DNA from the Nicotiana species noted above, and also several others from within the genus, suggested that QPRTase is encoded by a small gene family in all the species investigated.
Similar content being viewed by others
References
Baldwin, I.T. 1988. Damage induced alkaloids in tobacco: pot bound plants are not inducible. J. Chem. Ecol. 14: 1113–1120.
Baldwin, I.T. and Ohnmeiss T.E. 1993. Alkaloid responses to damage in Nicotiana native to North America. J. Chem. Ecol. 19: 1143–1153.
Baldwin, I.T. and Preston, C.A. 1999. The eco-physiological complexity of plant responses to insect herbivores. Planta 208: 137–145.
Baldwin, I.T. and Schmelz, E.A. 1994. Constraints on an induced defense: the role of leaf area. Oecologia 97: 424–430.
Baldwin, I.T., Schmelz, E.A. and Ohnmeiss, T.E. 1994. Woundinduced changes in root and shoot jasmonic acid pools correlate with induced nicotine synthesis in Nicotiana sylvestris Spegazzini and Comes. J. Chem. Ecol. 20: 2139–2157.
Baldwin, I.T., Zhang, Z.-P., Diab, N., Ohnmeiss, T.E., McCloud, E.S., Lynds, G.Y. and Schmelz, E.A. 1997. Quantifications, correlations and manipulations of wound-induced changes in jasmonic acid and nicotine in Nicotiana sylvestris. Planta 201: 397–407.
Bhatia, R. and Calvo, K.C. 1995. The sequencing, expression, purification, and steady-state kinetic analysis of quinolinate phosphoribosyl transferease from Escherichia coli. Arch. Biochem. Biophys. 2: 270–278.
Castorena, J.L., Garriott, J.C., Barnhardt, F.E. and Shaw, R.F. 1987. A fatal poisoning from Nicotiana glauca. Clin. Toxicol. 25: 429–435.
Chaplin, J.F. 1975. Registration of LAFC53 tobacco germplasm (Reg. #GP13). Crop Sci. 15: 282.
Chaplin, J. F. 1986. Registration of MAFC 5 tobacco germplasm. Crop Sci. 6: 214.
Chang, H.-K. and Zylstra, G.J. 1999. Role of quinolinate phosphoribosyl transferase in degradation of phthalate by Burkholderia cepacia DBO1. J. Bact. 181: 3069–3075.
Cherep, N.N. and Kormanitskii, I.K. 1991. Mitochondrial Nicotiana genome [Russian]. Biopol. i lekta 7: 45–50.
Chung, C.T. and Miller, R.H. 1988. A rapid and convenient method for the preparation and storage of competent bacterial cells. Nucl. Acids Res. 16: 3580.
Dawson, R.F. 1941. The localization of the nicotine synthetic mechanism in the tobacco plant. Science 94: 396–397.
Dawson, R.F. 1942. Accumulation of nicotine in reciprocal grafts of tomato and tobacco. Am. J. Bot. 29: 66–71.
Dawson, R.F. 1945. An experimental analysis of alkaloid production in Nicotiana: the origin of nornicotine. Am. J. Bot. 32: 416–423.
Dawson, R.F. 1962. Biosynthesis of the Nicotiana alkaloids. In: W.R. Brode (Ed.) Science in Progress, vol. 12, Yale University Press, New Haven, CT, pp. 117–143.
Eads, J.C., Ozturk, D., Wexler, T.B., Grubmeyer, C. and Sacchettini, J.C. 1997. A new function for a common fold: the crystal structure of quinolinic acid phosphoribosyltransferase. Structure 15: 47–58.
Fecker, L.F., Ruegenhagen, C. and Berlin, J. 1993. Increased production of cadaverine and anabasine in hairy root cultures of Nicotiana tabacum expressing a bacterial lysine decarboxylase gene. Plant Mol. Biol. 23: 11–21.
Feth, F., Wagner, R. and Wagner, K.G. 1986. Regulation in tobacco callus of enzyme activities of the nicotine pathway. 1. The route ornithine to methylpyrroline. Planta 168: 402–407.
Fukuoka, S.-I., Nyaruhucha, C.M. and Shibata, K. 1998. Characterization and functional expression of the cDNA encoding human brain quinolinate phosphoribosyltransferase. Biochim. Biophys. Acta 1395: 192–201.
Gallie, D.R. 1996. Translational control of cellular and viral mRNAs. Plant Mol. Biol. 32: 145–158.
Goodspeed, T.H. and Thompson, M.C. 1959. Cytotaxonomy of Nicotiana. II. Bot. Rev. 25: 385–415.
Hamill J.D., Parr, A.J., Robins R.J. and Rhodes, M.J.C. 1986. Secondary product formation by cultures of Beta vulgaris and Nicotiana rustica transformed with Agrobacterium rhizogenes. Plant Cell Rep. 5: 111–114.
Hamill, J.D. and Lidgett, A. 1997. Hairy root cultures: opportunities and key protocols for studies in metabolic engineering. In: P.M. Doran (Ed.) Hairy Roots: Culture and Applications, Harwood Academic Publishers, Amsterdam, pp. 1–29.
Hashimoto, T. and Yamada, Y. 1994. Alkaloid biogenesis: molecular aspects. Annu. Rev. Plant Physiol. Plant Mol. Biol. 45: 257–285.
Hashimoto, T., Shoji, T., Mihara, T., Oguri, H., Tamaki, K., Suzuki, K.-I. and Yamada, Y. 1998a. Intraspecific variability of the tandem repeats in Nicotiana putrescine N-methyltransferases. Plant Mol. Biol. 37: 25–37.
Hashimoto, T., Tamaki, K., Suzuki, K.-I. and Yamada, Y. 1998b. Molecular cloning of plant spermidine synthases. Plant Cell Physiol. 39: 73–79.
Herminghaus, S., Tholl, D., Ruegenhagen, C., Fecker, L.F., Leuschner, C. and Berlin, J. 1996. Improved metabolic action of a bacterial lysine decarboxylase gene in tobacco hairy root cultures by its fusion to a rbcS transit peptide coding sequence. Transgenic Res. 5: 193–201.
Hibi, N., Fujita, T., Hatano, M., Hashimoto, T. and Yamada, Y. 1992. Putrescine N-methyltransferase in cultured roots of Hyoscyamus albus. Plant Physiol. 100: 826–835.
Hibi, N., Higashiguchi, S., Hashimoto, T. and Yamada, Y. 1994. Gene expression in tobacco low-nicotine mutants. Plant Cell 6: 723–735.
Hughes, K.T., Dessen, A., Gray, J.P. and Grubmeyer, C. 1993. The Salmonella typhimurium nadC gene: sequence determination by use of Mud-P22 and purification of quinolinate phosphoribosyltransferase. J. Bact. 175: 479–486.
Imanishi, S., Hashizume, K., Nakita, M., Kojima, H., Matsubayashi, Y., Hashimoto, T., Yamada, Y. and Nakamura, K. 1998. Differential induction by methyl jasmonate of genes encoding ornithine decarboxylase and other enzymes involved in nicotine biosynthesis in tobacco cell cultures. Plant Mol. Biol. 38: 1101–1111.
Jamet, E., Durr, A. and Fleck, J. 1987. Absence of some truncated genes in the amphidiploid Nicotiana tabacum. Gene 59: 213–221.
Jinks, J.L., Caligari, P.D.S. and Ingram, N.R. 1981. Gene transfer in Nicotiana rustica using irradiated pollen. Nature 291: 586–588.
Joshi, C.P., Zhou, H., Huang, X. and Chiang, V. 1997. Context sequences of translation initiation codon in plants. Plant Mol. Biol. 35: 993–1001.
Karban, R. and Baldwin, I.T. 1997. Induced Responses to Herbivory. University of Chicago Press, Chicago.
Kozak, M. 1986. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryote ribosomes. Cell 44: 283–292.
Kozac, M. 1995. Adherance to the first-AUG rule when a second AUG codon follows closely upon the first. Proc. Natl. Acad. Sci. USA 92: 2662–2666.
Kutchan, T.M. 1995. Alkaloid biosynthesis: the basis for metabolic engineering of medicinal plants. Plant Cell 7: 1059–1070.
Leete, E. 1979. The alkaloids: alkaloids derived from ornithine, lysine and nicotinic acid. In: E.A. Bell and B.V. Charlwood (Eds.) Encyclopedia of Plant Physiology, New Series, vol. 8, Secondary Plant Products, Springer-Verlag, Berlin, pp. 65–91.
Legg, P.D. and Collins, G.B. 1971. Inheritance of percent total alkaloids in Nicotiana tabacum L. II. Genetic effects of two loci in Burley 21 × LA Burley 21 population. Can. J. Genet. Cytol. 13: 287–291.
Lidgett, A.J., Moran, M., Wong, K.A.L., Furze, J., Rhodes, M.J.C. and Hamill, J.D. 1995. Isolation and expression pattern of a cDNA encoding a cathepsin B-like protease from Nicotiana rustica. Plant Mol. Biol. 29: 379–384.
Lin, X., Kaul, S., Rounsley, S., Shea, T.P., Benito, M.-I., Town, C.D., Fujil, C.Y., Mason, T., Bowman, C.L., Barnstead, M., Feldblum, T.V., Buell, C.R., Ketchum, K.A., Lee, J., Ronning, C.M., Koo, H.L., Moffat, K.S., Cronin, L.A., Shen, M., Pai, G., van Aken, S., Umayam, L., Tallon, L.J., Gill, J.E., Adams, M.D., Carrera, A.J., Creasy, T.H., Goodman, H.M., Somerville, C.R., Copenhaver, G.P., Preuss, D., Nierman, W.C., White, O., Eisen, J.A., Salzburg, S.L., Fraser, C.M. and Venter, J.C. 1999. Sequence and analysis of chromosome 2 of the plant Arabidopsis thaliana. Nature 402: 761–768.
Mason, J. 1990. Commercial Hydroponics. Kangaroo Press, Kenthurst, Australia.
Mellick, L.B., Makowski, T., Mellick, G.A. and Borger, R. 1999. Neuromuscular blockade after ingestion of tree tobacco (Nicotiana glauca). Ann. Emerg. Med. 34: 101–104
Mizusaki, S., Tanebe, Y., Noguchi, M. and Tamaki, E. 1973. Changes in the activities of ornithine decarboxylase, putrescine N-methyltransferase and N-methylputrescine oxidase in tobacco roots in relation to nicotine biosynthesis. Plant Cell Physiol. 14: 103–110.
Nakai, K. and Kanehisa, M. 1992. A knowledge base for predicting protein localization sites in eukaryotic cells. Genomics 14: 897–911. (http://psort.nibb.ac.jp:8800.)
Ohnmeiss, T.E., McCloud, E.S., Lynds, G.Y. and Baldwin, I.T. 1997. Within plant relationships among wounding, jasmonic acid, and nicotine: implications for defence in Nicotiana sylvestris. New Phytol. 137: 441–452.
Parr, A.J. and Hamill, J.D. 1987. Relationship between Agrobacterium rhizogenes transformed hairy roots and intact, uninfected Nicotiana plants. Phytochemistry 26: 3241–3245.
Pedersen, A.G. and Nielsen, H. 1997. Neural network prediction of translation initiation sites in eukaryotes: perspectives for EST and genome analysis. Proceedings 5th International Conference on Intelligent Systems in Molecular Biology (ISMB97). (http://www.cbs.dtu.dk/services/Netstart)
Riechers, D.E. and Timko, M.P. 1999. Structure and expression of the gene family encoding putrescine N-methyltransferase in Nicotiana tabacum: new clues to the evolutionary origin of cultivated tobacco. Plant Mol. Biol. 41: 387–401.
Rothnie, H. M. 1996. Plant mRNA 3'-end formation. Plant Mol. Biol. 32: 43–61.
Sambrook, J., Fritsch, E.F. and Maniatis T. 1989. Molecular Cloning: A Laboratory Manual, 2nd ed. Cold Spring Harbor Laboratory Press, Plainview, NY.
Saunders, J.W. and Bush, L.P. 1979. Nicotine biosynthetic enzyme activities in Nicotiana tabacum L. genotypes with different alkaloid levels. Plant Physiol. 64: 236–240.
Saitoh, F., Noma, M. and Kawashima, N. 1985 The alkaloid contents of sixty Nicotiana species. Phytochemistry 24: 477–480.
Sharma, V., Grubmeyer, C. and Sacchettini, J.C. 1998. Crystal structure of quinolinic acid phosphoribosyltransferase from Mycobacterium tuberculosis: a potential drug target. Structure 6: 1587–1599.
Sisson, V.A. and Severson, R.F. 1990. Alkaloid composition of the Nicotiana species. Beitr. Tabakforsch. Int. 14: 327–339.
Sjoeling, S. and Glaser, E. 1998. Mitochondrial targeting peptides in plants. Trends Plant Sci. 3: 136–140.
Soltis, P.S. and Soltis, D.E. 1995. The dynamic nature of polyploid genomes. Proc. Natl. Acad. Sci. USA. 92: 8089–8091.
Song, K., Lu, P., Tang, K. and Osborn, P.C. 1995. Rapid genome change in synthetic polyploids of Brassica and its implications for polylpoid evolution. Proc. Natl. Acad. Sci. USA. 92: 7719–7723.
Suzuki, K.-i., Yamada, Y. and Hashimoto T. 1999. Expression of Atropa belladonna putrescine N-methyltransferase gene in root pericycle. Plant Cell Physiol. 40: 289–297.
Volkov, R.A., Borisjuk, N.V., Panchuk, I.I., Schweizer, D. and Hemleben, V. 1999. Elimination and rearrangement of parental rDNA in the allotetraploid Nicotiana tabacum. Mol. Biol. Evol. 16: 311–320.
Wagner, R. and Wagner, K.G. 1985. The pyridine-nucleotide cycle in tobacco: enzyme activities for the de novo synthesis of NAD. Planta 165: 532–537.
Wagner, R., Feth, F. and Wagner, K.G. 1986a. Regulation in tobacco callus of enzyme activities of the nicotine pathway. II. The pyridine-nucleotide cycle. Planta 168: 408–413
Wagner, R., Feth, F. and Wagner, K. G. 1986b. The regulation of enzyme activities of the nicotine pathway in tobacco. Physiol. Plant. 68: 667–672.
Wagner, R., Feth, F. and Wagner, K. G. 1986c. The pyridine nucleotide cycle in tobacco. Enzyme activities for the recycling of NAD. Planta 167: 226–232
Walton, N.J. and Belshaw, N.J. 1988. The effect of cadaverine on the formation of anabasine from lysine in hairy root cultures of Nicotiana hesperis. Plant Cell Rep. 7: 115–118.
Walton, N.J., Robins, R.J. and Rhodes, M.J.C. 1988. Perturbation of alkaloid production by cadaverine in hairy root cultures of Nicotiana rustica. Plant Sci. 54: 125–131.
Wink, M. 1988. Plant breeding: importance of plant secondary metabolites for protection against pathogens and herbivores. Theor. Appl. Genet. 75: 225–233.
Wink, M. 1997. Special nitrogen metabolism. In: P.M. Dey and J.B. Harborne (Eds.) Plant Biochemistry, Academic Press, San Diego, CA.
Yates, R.A. and Pardee, A.R. 1956. Pyrimidine biosynthesis in Escherichia coli. J. Biol. Chem. 221: 743–756.
Zhang, Z.-P. and Baldwin, I.T. 1997. Transport of [2-14C] jasmonic acid from leaves to roots mimics wound-induced changes in endogenous jasmonic acid pools in Nicotiana sylvestris. Planta 203: 436–441.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sinclair, S.J., Murphy, K.J., Birch, C.D. et al. Molecular characterization of quinolinate phosphoribosyltransferase (QPRTase) in Nicotiana. Plant Mol Biol 44, 603–617 (2000). https://doi.org/10.1023/A:1026590521318
Issue Date:
DOI: https://doi.org/10.1023/A:1026590521318