Abstract
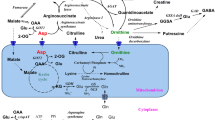
Leucine and α-ketoisocaproate (α-KIC) were perfused at increasing concentrations into rat brain hippocampus by microdialysis to mimic the conditions of maple syrup urine disease. The effects of elevated leucine or α-KIC on the oxidation of L-[U-14C]glutamate and L-[U-14C]glutamine in the brain were determined in the non-anesthetized rat. 14CO2 generated by the metabolic oxidation of [l4C]glutamate and [14C]glutamine in brain was measured following its diffusion into the eluant during the microdialysis. Leucine and α-KIC exhibited differential effects on 14CO2 generation from radioactive glutamate or glutamine. Infusion of 0.5 mM α-KIC increased [l4C]glutamate oxidation approximately 2-fold; higher concentrations of α-KIC did not further stimulate [14C]glutamate oxidation. The enhanced oxidation of [14C]glutamate may be attributed to the function of α-KIC as a nitrogen acceptor from [14C]glutamate yielding [14C]α-ketoglutarate, an intermediate of the tricarboxylic acid cycle. [14C-]glutamine oxidation was not stimulated as much as [14C-]glutamate oxidation and only increased at 10 mM α-KIC reflecting the extra metabolic step required for its oxidative metabolism. In contrast, leucine had no effect on the oxidation of either [14C]glutamate or [14C]glutamine. In maple syrup urine disease elevated α-KIC may play a significant role in altered energy metabolism in brain while leucine may contribute to clinical manifestations of this disease in other ways.
Similar content being viewed by others
REFERENCES
Tanaka, K., and Rosenberg, L. E. 1983. Disorders of branched chain amino acid and organic acid metabolism, Pages 440–473, in Stanbury, J. B., Wyngaarden, J. B., Fredrickson, D. S., Goldstein, J. L., and Brown, M. S. (eds.), The Metabolic Basis of Inherited Disease, Fifth edition, McGraw Hill Book Co., New York.
Tanaka, K. 1986. Inborn errors of branched-chain amino acid metabolism, Pages 201–282, in Odessey. R. (ed), Problems and Potential of Branched-Chain Amino Acids in Physiology and Disease, Elsevier Publishers B. V., Amsterdam.
Yuwiler, A., and Geller, E. 1965. Serotonin depletion by dietary leucine. Nature 208:83–84.
Tashian, R. E. 1961. Inhibition of brain glutamic acid decarboxylase by phenylalanine, leucine, and valine derivatives: A suggestion concerning the neurological defect in phenylketonuria and branched-chain keto aciduria. Metabolism 10:393–402.
Halestrap, A. P., Brand, M. D., and Denton, R. M. 1974. Inhibition of mitochondrial pyruvate transport by phenylpyruvate and α-ketoisocaproate. Biochim. Biophys. Acta 367:102–108.
Land, J. M., Mowbray, J., and Clark, J. B. 1974. Control of pyruvate and β-hydroxybutyrate utilization in rat brain mitochondria and its relevance to phenylketonuria and maple syrup urine disease. J. Neurochem. 26:823–830.
Yielding, K. L., and Tomkins, G. M. 1961. An effect of L-leucine and other essential amino acids on the structure and activity of glutamic dehydrogenase. Proc. Natl. Acad. Sci., USA 47:983–990.
Coueé, I., and Tipton, K. F. 1989. Activation of glutamate dehydrogenase by L-leucine. Biochim. Biophys. Acta. 995:97–101.
Erecińska, M., and Nelson, D. 1990. Activation of glutamate dehydrogenase by leucine and its nonmetabolizable analogue in rat brain synaptosomes. J. Neurochem. 54:1335–1343.
Yudkoff, M., Daikhin, Y., Nissim, I., Pleasure, D., Stern, J., and Nissim, I. 1994. Inhibition of astrocyte glutamine production by α-ketoisocaproic acid. J. Neurochem. 63:1508–1515.
Zielke, H. R., Huang, Y., Tildon, J. T., Zielke, C. L., and Baab, P. J. 1996. Elevation of amino acids in the interstitial space of the rat brain following infusion of large neutral amino and keto acids by microdialysis: leucine infusion. Dev. Neurosci. 18:415–419.
Zielke, H. R., Huang, Y., Tildon, J. T., Zielke, C. L., and Baab, P. J. 1996. Elevation of amino acids in the interstitial space of the rat brain following infusion of large neutral amino and keto acids by microdialysis: alpha-ketoisocaproate infusion. Dev. Neurosci. 18:420–425.
Yudkoff, M., Daikhin, Y., Lin, Z., Nissim, I., Stern, J., Pleasure, D., and Nissim, I. 1994. Relationships of leucine and glutamate metabolism in cultured astrocytes. J. Neurochem. 62:1192–1202.
Huang, Y., Zielke, C. L., Tildon, J. T., and Zielke, H. R. 1993. Monitoring in vivo oxidation of 14C-labelled substrates to 14CO2 by brain microdialysis. Dev. Neurosci. 15:233–239.
Sendelbeck, L. 1987. Recipe for preparation of artificial CSF, Page 4, in Ray, N. (ed.), Special Delivery, vol. 8(3), ALZA Corp., Palo Alto, CA.
Perry, T. L. 1988. Glutamine, glutamate, and GABA in human diseases, Pages 113–125, in Kvamme, E. (ed.), Glutamine and Glutamate in Mammals, Vol. II, CRC Press, Boca Raton, FL.
Benveniste, H., and Hütemeier, P. C. 1990. Microdialysis—theory and application. Prog. Neurobiol. 35:195–215.
Itoh, T., and Quastel, J. H. 1970. Acetoacetate metabolism in infant and adult rat brain in vitro. Biochem. J. 116:641–655.
Williamson, A. M., and Robinson, D. H. 1980. Physiological roles of ketone bodies as substrates and signals in mammalian tissues. Physiol. Rev. 60:143–189.
Cremer, J. E. 1981. Nutrients for the brain: problems in supply. Early Hum. Develop. 5:117–132.
Roeder, L. M., Tildon, J. T., and Holman, D. C. 1984. Competition among oxidizable substrates in brains of young and adult rats. Dissociated cells. Biochem. J. 219:131–135.
Tildon, J. T. 1983. Glutamine: a possible energy source for the brain, Pages 415–430, in Hertz, L., Kvamme, E., McGeer, E. G., and Schousboe, A. (eds.), Glutamine, Glutamate and GABA in the Central Nervous System, Alan R Liss, Inc., New York.
Butterworth, R. F. 1983. Metabolism of glutamate and related amino acids in insulin hypoglycemia, Pages 595–608, in Hertz, L., Kvamme, E., McGeer, E. G., and Schousboe, A. (eds.), Glutamine, Glutamate and GABA in the Central Nervous System, Alan R Liss, Inc., New York.
Yu, A. C., Schousboe, A., and Hertz, L. 1982. Metabolic fate of 14C-labeled glutamate in astrocytes in primary cultures. J. Neurochem. 39:954–960.
Yu, A. C. H., Fisher, T. E., Hertz, E., Tildon, J. T., Schousboe, A., and Hertz, L. 1984. Metabolic fate of [14C]-glutamine in mouse cerebral neurons in primary cultures. J. Neurosci. Res. 11:351–357.
Brookes, N. 1992. Effect of intracellular glutamine on the uptake of large neutral amino acids in astrocytes: concentrative Na+independent transport exhibits metastability. J. Neurochem. 59: 227–235.
Cangiano, C., Cardelli-Cangiano, P., James, J. H., Rossi-Fanelli, F., Patrizi, M. A., Brackett, K. A., Strom, R., and Fischer, J. E. 1983. Brain microvessels take up large neutral amino acids in exchange for glutamine: cooperative role of Na+dependent and Na+independent systems. J. Biol. Chem. 258:8949–8954.
Hamberger, A. C., Chiang, G. H., Nylen, E. S., Scheft, S. W., and Cotman, C. W. 1979. Glutamate as a CNS transmitter. I. Evaluation of glucose and glutamine as precursor for the synthesis of preferentially released glutamate. Brain Res. 168:513–530.
Battaglioli, G., and Martin, D. L. 1990. Stimulation of synaptosomal γ-aminobutyric acid synthesis by glutamate and glutamine. J. Neurochem. 54:1179–1187.
Yudkoff, M., Nissim, I., Daikhin, Y., Lin, Z.-P., Nelson, D., Pleasure, D., and Erecinska, M. 1993. Brain glutamate metabolism: neuronal-astroglial relationships. Dev. Neurosci. 15:343–350.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zielke, H.R., Huang, Y., Baab, P.J. et al. Effect of α-Ketoisocaproate and Leucine on the in Vivo Oxidation of Glutamate and Glutamine in the Rat Brain. Neurochem Res 22, 1159–1164 (1997). https://doi.org/10.1023/A:1027325620983
Issue Date:
DOI: https://doi.org/10.1023/A:1027325620983