Abstract
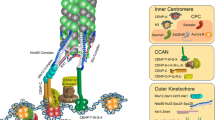
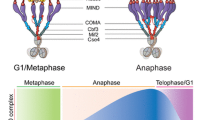
Kinetochores are proteinaceous organelles that assemble on centromeric DNA to direct chromosome segregation in all eukaryotes. While many aspects of kinetochore function are conserved, the nature of the chromosomal domain upon which kinetochores assemble varies dramatically between different species. In monocentric eukaryotes, kinetochores assemble on a localized region of each chromosome. In contrast, holocentric species such as the nematode Caenorhabditis elegans have diffuse kinetochores that form along the entire length of their chromosomes. Here, we discuss the nature of chromosome segregation in C. elegans. In addition to reviewing what is known about kinetochore function, chromosome structure, and chromosome movement, we consider the consequences of the specialized holocentric architecture on chromosome segregation.
Similar content being viewed by others
References
Albertson DG, Thomson JN (1982) The kinetochores of Caenorhabditis elegans. Chromosoma 86: 409–428.
Albertson DG, Thomson JN (1993) Segregation ofholocentric chromosomes at meiosis in the nematode, Caenorhabditis elegans. Chromosome Res 1: 15–26.
Blower MD, Sullivan BA, Karpen GH (2002) Conserved organization ofcentromeric chromatin in flies and humans. Dev Cell 2: 319–330.
Buchwitz BJ, Ahmad K, Moore LL, Roth MB, Henikoff S (1999) A histone-H3-like protein in C. elegans. Nature 401: 547–548.
Chan RC, Chan A, Jeon M et al. (2003) Chromosome cohesion is regulated by a clock gene paralogue TIM-1. Nature 424: 1002–1009.
Cheeseman IM, Desai A (2004) Cell division: feeling tense enough? Nature 428: 32–33.
Cleveland DW, Mao Y, Sullivan KF (2003) Centromeres and kinetochores: from epigenetics to mitotic checkpoint signaling. Cell 112: 407–421.
Comings DE, Okada TA (1971) Fine structure ofkinetoch ore in Indian muntjac. Exp Cell Res 67: 97–110.
Comings DE, Okada TA (1972) Holocentric chromosomes in Oncopeltus: kinetochore plates are present in mitosis but absent in meiosis. Chromosoma 37: 177–192.
Dernburg AF (2001) Here, there, and everywhere: kinetochore function on holocentric chromosomes. J Cell Biol 153: F33–F38.
Desai A, Rybina S, Muller-Reichert T et al. (2003) KNL-1 directs assembly ofthe microtubule-binding interface of the kinetochore in C. elegans. Genes Dev 17: 2421–2435.
Flemming W (1880) Beiträge zur Kenntnis der Zelle und ihrer Lebenserscheinungen. Arch Mikrosk Anat 18: 151–259.
Gonczy P, Echeverri G, Oegema K et al. (2000) Functional genomic analysis ofcell division in C. elegans using RNAi of genes on chromosome III. Nature 408: 331–336.
Goshima G, Kiyomitsu T, Yoda K, Yanagida M (2003) Human centromere chromatin protein hMis12, essential for equal segregation, is independent ofCENP-A loading pathway. J Cell Biol 160: 25–39.
Grill SW, Gonczy P, Stelzer EH, Hyman AA (2001) Polarity controls forces governing asymmetric spindle positioning in the Caenorhabditis elegans embryo. Nature 409: 630–633.
Hagstrom KA, Holmes VF, Cozzarelli NR, Meyer BJ (2002) C. elegans condensin promotes mitotic chromosome architecture, centromere organization, and sister chromatid segregation during mitosis and meiosis. Genes Dev 16: 729–742.
Hillers KJ, Villeneuve AM (2003) Chromosome-wide control ofmeiotic crossing over in C. elegans. Curr Biol 13: 1641–1647.
Howe M, McDonald KL, Albertson DG, Meyer BG (2001) HIM-10 is required for kinetochore structure and function on Caenorhabditis elegans holocentric chromosomes. J Cell Biol 153: 1227–1238.
Howman EV, Fowler KJ, Newson AJ et al. (2000) Early disruption ofcentromeric chromatin organization in centromere protein A (Cenpa) null mice. Proc NatlAcad Sci USA 97: 1148–1153.
Hsu JY, Sun ZW, Li X et al. (2000) Mitotic phosphorylation ofhistone H3 is governed by Ipl1/aurora kinase and Glc7/ PP1 phosphatase in budding yeast and nematodes. Cell 102: 279–291.
Kaitna S, Mendoza M, Jantsch-Plunger V, Glotzer M (2000) Incenp and an aurora-like kinase form a complex essential for chromosome segregation and efficient completion of cytokinesis. Curr Biol 10: 1172–1181.
Kitagawa R, Rose AM (1999) Components ofthe spindleassembly checkpoint are essential in Caenorhabditis elegans. Nat Cell Biol 1: 514–521.
Maddox P, Straight A, Coughlin P, Mitchison TJ, Salmon ED (2003) Direct observation ofmicrotubule dynamics at kinetochores in Xenopus extract spindles: implications for spindle mechanics. J Cell Biol 162: 377–382.
Marshall WF, Straight A, Marko JF et al. (1997) Interphase chromosomes undergo constrained diffusional motion in living cells. Curr Biol 7: 930–939.
McAinsh AD, Tytell JD, Sorger PK (2003) Structure, function, and regulation ofbudding yeast kinetochores. Annu Rev Cell Dev Biol 19: 519–539.
McEwen BF, Hsieh CE, Mattheyses AL, Rieder CL (1998) A new look at kinetochore structure in vertebrate somatic cells using high-pressure freezing and freeze substitution.Chromosoma 107: 366–375.
Mito Y, Sugimoto A, Yamamoto M (2003) Distinct developmental function of two Caenorhabditis elegans homologs ofthe cohesin subunit Scc1/Rad21. MolBiolCel l 14: 2399–2409.
Moore LL, Roth MB (2001) HCP-4, a CENP-C-like protein in Caenorhabditis elegans, is required for resolution of sister centromeres. J Cell Biol 153: 1199–1208.
Moore LL, Morrison M, Roth MB (1999) HCP-1, a protein involved in chromosome segregation, is localized to the centromere ofmitotic chromosomes in Caenorhabditis elegans. J Cell Biol 147: 471–480.
Nystul TG, Goldmark JP, Padilla PA, Roth MB (2003) Suspended animation in C. elegans requires the spindle checkpoint. Science 302: 1038–1041.
Oegema K, Desai A, Rybina S, Kirkham M, Hyman AA (2001) Functional analysis ofkinetocho re assembly in Caenorhabditis elegans. J Cell Biol 153: 1209–1225.
Ono T, Losada A, Hirano M, Myers MP, Neuwald AF, Hirano T (2003) Differential contributions of condensin I and condensin II to mitotic chromosome architecture in vertebrate cells. Cell 115: 109–121.
O'Toole ET, McDonald KL, Mantler J, McIntosh JR, Hyman AA, Muller-Reichert T (2003) Morphologically distinct microtubule ends in the mitotic centrosome of Caenorhabditis elegans. J Cell Biol 163: 451–456.
Pasierbek P, Jantsch M, Melcher M, Schleiffer A, Schweizer D, Loidl J (2001) A Caenorhabditis elegans cohesion protein with functions in meiotic chromosome pairing and disjunction. Genes Dev 15: 1349–1360.
Pasierbek P, Fodermayr M, Jantsch V, Jantsch M, Schweizer D, Loidl J (2003) The Caenorhabditis elegans SCC-3 homologue is required for meiotic synapsis and for proper chromosome disjunction in mitosis and meiosis. Exp Cell Res 289: 245–255.
Pazy B, Plitmann, U(2002) New perspectives on the mechanisms ofchromoso me evolution in parasitic flowering plants. Bot J Linnean Soc 138: 117–122.
PimpinelliS, GodayC(1989)Unusualkinetochoresandchromatin diminution in Parascaris. Trends Genet 5: 310–315.
Rieder CL (1982) The formation, structure, and composition ofthe mammalian kinetochore and kinetochore fiber. Int Rev Cytol 79: 1–58.
Rieder CL, Salmon ED (1998) The vertebrate cell kinetochore and its roles during mitosis. Trends Cell Biol 8: 310-318.
Romano A, Guse A, Krascenicova I, Schnabel H, Schnabel R, Glotzer M (2003) CSC-1: a subunit ofthe Aurora B kinase complex that binds to the survivin-like protein BIR-1 and the incenp-like protein ICP-1. J Cell Biol 161: 229–236.
Scaerou F, Starr DA, Piano F, Papoulas O, Karess RE, Goldberg ML (2001) The ZW10 and Rough Deal checkpoint proteins function together in a large, evolutionarily conserved complex targeted to the kinetochore. J Cell Sci 114: 3103–3114.
Skibbens RV, Rieder CL, Salmon ED (1995) Kinetochore motility after severing between sister centromeres using laser microsurgery: evidence that kinetochore directional instability and position is regulated by tension. J Cell Sci 108(Pt 7): 2537-2548.
Speliotes EK, Uren A, Vaux D, Horvitz HR (2000) The survivin-like C. elegans BIR-1 protein acts with the Auroralike kinase AIR-2 to affect chromosomes and the spindle midzone. MolCel l 6: 211–223.
Starr DA, Williams BC, Li Z, Etemad-Moghadam B, Dawe RK, Goldberg ML (1997) Conservation ofthe centromere/kinetochore protein ZW10. J Cell Biol 138: 1289–1301.
Stear JH, Roth MB (2002) Characterization ofHCP-6, a C. elegans protein required to prevent chromosome twisting and merotelic attachment. Genes Dev 16: 1498–1508.
Sullivan KF (2001) A solid foundation: functional specialization ofcentromeric chromatin. Curr Opin Genet Dev 11: 182–188.
Sullivan BA, Blower MD, Karpen GH (2001) Determining centromere identity: cyclical stories and forking paths. Nat Rev Genet 2: 584–596.
Swedlow JR, Hirano T (2003) The making ofthe mitotic chromosome: modern insights into classical questions. Mol Cell 11: 557–569.
Van Hooser AA, Ouspenski II, Gregson HC et al. (2001) Specification ofkinetocho re-forming chromatin by the histone H3 variant CENP-A. J Cell Sci 114: 3529–3542.
Wang F, Yoder J, Antoshechkin I, Han M (2003) Caenorhabditis elegans EVL-14/PDS-5 and SCC-3 are essential for sister chromatid cohesion in meiosis and mitosis. MolCel l Biol 23: 7698–7707.
Waters JC, Chen RH, Murray AW, Salmon ED (1998) Localization ofMad2 to kinetochores depends on microtubule attachment, not tension. J Cell Biol 141: 1181–1191.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Maddox, P.S., Oegema, K., Desai, A. et al. "Holo"er than thou: Chromosome segregation and kinetochore function in C. elegans . Chromosome Res 12, 641–653 (2004). https://doi.org/10.1023/B:CHRO.0000036588.42225.2f
Issue Date:
DOI: https://doi.org/10.1023/B:CHRO.0000036588.42225.2f