Abstract
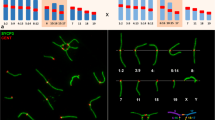
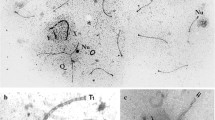
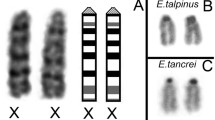
Mice that are double heterozygous for the semi-identical T(1;13)70H and T(1;13)1Wa reciprocal translocations display a great variation in male fertility. The synaptic behaviour of the different translocation chromosomes of adult males was studied in relation to this parameter. Juvenile males and embryonic females (16 and 18 days old) were included for comparison. In agreement with the minor differences in the translocation breakpoint positions, two differently sized heteromorphic bivalents are formed in meiotic prophase of both sexes (a quadrivalent was never encountered). Synaptonemal complex (SC) configurations of both bivalents in either sex are characterized by a high degree of non-homologous synapsis at zygotene-early pachytene. The rate of synaptic adjustment during pachytene is dependent on the size of the heteromorphic bivalent and varies between the sexes. Differences in SC configuration and morphology of the small heteromorphic bivalent in particular exist between the sexes and between animals. In males, this correlates with different degrees of fertility. Normal SC morphology in a fully synapsed small heteromorphic bivalent is an important determinant of successful meiosis and spermatogenesis. Moreover, aberrant synapsis favours the ‘unsaturated pairing site’ model as the primary cause for male sterility.
Similar content being viewed by others
References
Ashley T (1984) Application of the spreading techniques to structural heterozygotes. In: Bennett MD, Gropp A, Wolf U, eds. Chromosomes Today. London: George Allen & Unwin Ltd, pp 80–89.
Ashley T (1988) G-band position effects on meiotic synapsis and crossing over. Genetics 118: 307–317.
Ashley T (1990) Prediction of mammalian meiotic synaptic and recombinational behavior of inversion heterozygotes based on mitotic breakpoint data and the possible evolutionary consequences. Genetica 83: 1–7.
Ashley T, Cacheiro NL (1990) Correlation between meiotic behavior and breakpoints with respect to G-bands in two X-4 mouse translocations: T(X;4)7R1 and T(X;4)8R1. Cytogenet Cell Genet 53: 178–184.
Ashley T, Moses MJ, Solari AJ (1981) Fine structure and behaviour of a pericentric inversion in the sand rat, Psammomys obesus. J Cell Sci 50: 105–119.
Ashley T, Ried T, Ward DC (1994) Detection of nondisjunction and recombination in meiotic and postmeiotic cells from XYSxr [XY,Tp(Y)1Ct]mice using multicolor fluorescence in situ hybridization. Proc Natl Acad Sci USA 91: 524–528.
Ashley T, Plug AW, Xu J, et al. (1995) Dynamic changes in Rad51 distribution on chromatin during meiosis in male and female vertebrates. Chromosoma 104: 19–28.
Barlow C, Hirotsune S, Paylor R, et al. (1996) Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell 86: 159–171.
Bishop DK (1994) RecA homologs Dmc1 and Rad51 interact to form multiple nuclear complexes prior to meiotic chromosome synapsis. Cell 79: 1081–1092.
Borodin PM, Gorlov IP, Ladygina TY (1992) Synaptic interrelationships between the segments of the heteromorphic bivalent in double heterozygotes for paracentric inversions in chromosome 1 of the house mouse Chromosoma 101: 374–379.
Braun RE, Behringer RR, Peschon JJ, Brinster RL, Palmiter RD (1989) Genetically haploid spermatids are phenotypically diploid. Nature 337: 373–376.
Burgoyne PS, Baker TG (1985) Perinatal oocyte loss in XO mice and its implications for the aetiology of gonadal dysgenesis in XO women. J Reprod Fertil 75: 633–645.
Burgoyne PS, Mahadevaiah S, Mittwoch U (1985) A reciprocal autosomal translocation which causes male sterility in the mouse also impairs oogenesis. J Reprod Fertil 75: 647–652.
de Boer P, Searle AG (1980) Summary and synthesis. Workshop on chromosomal aspects of male sterility in mammals. J Reprod Fertil 60: 257–265.
de Boer P, Speed RM (1982) Meiosis of T70H translocation trisomic male mice. II Meiotic rate, spermatocyte interactions and fertility. Chromosoma 87: 315–325.
de Boer P, de Jong JH (1989) Chromosome pairing and fertility in mice. In: Gilles CB, ed. Fertility and Chromosome Pairing: Recent Studies in Plants and Animals. Boca Raton: CRC Press, pp 37–76.
de Boer P, Searle AG, van der Hoeven FA, de Rooij DG, Beechey CV (1986) Male pachytene pairing in single and double translocation heterozygotes and spermatogenic impairment in the mouse. Chromosoma 93: 326–336.
Dietrich AJ, de Boer P (1983) A sequential analysis of the development of the synaptonemal complex in spermatocytes of the mouse by electron microscopy using hydroxyurea and agar filtration. Genetica 61: 119–129.
Dietrich AJ, Mulder RJ (1983) A light-and electron microscopic analysis of meiotic prophase in female mice. Chromosoma 88: 377–385.
Dym M, Fawcett DW (1971) Further observations on the numbers of spermatogonia, spermatocytes, and spermatids connected by intercellular bridges in the mammalian testis. Biol Reprod 4: 195–215.
Forejt J (1974) Non-random association between a specific autosome and the X chromosome in meiosis of the male mouse: possible consequences of homologous centromeres' separation. Cytogenet Cell Genet 19: 369–383.
Forejt J (1996) Hybrid sterility in the mouse. Trends Genet 12: 412–417.
Forejt J, Gregorova S (1977) Meiotic studies of translocations causing male sterility in the mouse. I. Autosomal reciprocal translocations. Cytogenet Cell Genet 19: 159–179.
Forejt J, Gregorova S, Goetz P (1981) XY pair associates with the synaptonemal complex of autosomal male-sterile translocations in pachytene spermatocytes of the mouse (Mus musculus). Chromosoma 82: 41–53.
Gabriel-Robez O, Rumpler Y (1994) The meiotic pairing behaviour in human spermatocytes carrier of chromosome anomalies and their repercussions on reproductive fitness. I: Inversions and insertions. A European collaborative study. Ann Genet 37: 3–10.
Gabriel-Robez O, Rumpler Y (1996) The meiotic pairing behaviour in human spermatocytes carrier of chromosome anomalies and their repercussions on reproductive fitness. II: Robertsonian and reciprocal translocations. A European collaborative study. Ann Genet 39: 17–25.
Gillies CB (1989) Preface. In: Gilles CB, ed. Fertility and Chromosome Pairing: Recent Studies in Plants and Animals. Boca Raton: CRC Press.
Guitart M, Coll MD, Ponsa M, Egozcue J (1985) Sequential study of synaptonemal complexes in mouse spermatocytes by light and electron microscopy. Genetica 67: 21–30.
Hawley RS, Friend SH (1996) Strange bedfellows in even stranger places: the role of ATM in meiotic cells, lymphocytes, tumors, and its functional links to p53. Genes Dev 10: 2383–2388.
Keegan KS, Holtzman DA, Plug AW et al. (1996) The Atr and Atm protein kinases associate with different sites along meiotically pairing chromosomes. Genes Dev 10: 2423–2437.
Kluin PM, Kramer MF, de Rooij DG (1982) Spermatogenesis in the immature mouse proceeds faster than in the adult. Int J Androl 5: 282–294.
Lifschytz E, Lindsley DL (1972) The role of X-chromosome inactivation during spermatogenesis. Proc Natl Acad Sci USA 69: 182–186.
Mahadevaiah S, Mittwoch U, Moses MJ (1984) Pachytene chromosomes in male and female mice heterozygous for the Is(7;1)40H insertion. Chromosoma 90: 163–169.
Messier PE, Jean P, Richer CL (1986) Easy transfer of selected mitoses from light to electron microscopy. Cytogenet Cell Genet 43: 207–210.
Miklos GL (1974) Sex-chromosome pairing and male fertility. Cytogenet Cell Genet 13: 558–577.
Mittwoch U, Mahadevaiah SK (1992) Unpaired chromosomes at meiosis: cause or effect of gametogenic insufficiency? Cytogenet Cell Genet 59: 274–279.
Moses MJ (1980) New cytogenetic studies on mammalian meiosis. In: Serio M, Martini L, eds. Animal Models in Human Reproduction. New York: Raven Press, pp 169–190.
Moses MJ, Poorman PA (1981) Synaptonemal complex analysis of mouse chromosomal rearrangements. II. Synaptic adjustment in a tandem duplication. Chromosoma 81: 519–535.
Moses MJ, Dresser ME, Poorman PA (1984) Composition and role of the synaptonemal complex. Symp Soc Exp Biol 38: 245–270.
Peters AH, Plug AW, van Vugt MJ, de Boer P (1997) A drying-down technique for the spreading of mammalian meiocytes from the male and female germline. Chrom Res 5: 66–68
Plug AW, Xu J, Reddy G, Golub EI, Ashley T (1996) Presynaptic association of Rad51 protein with selected sites in meiotic chromatin. Proc Natl Acad Sci USA 93: 5920–5924.
Poorman PA, Moses MJ, Russell LB, Cacheiro NL (1981) Synaptonemal complex analysis of mouse chromosomal rearrangements. I. Cytogenetic observations on a tandem duplication. Chromosoma 81: 507–518.
Searle AG, Beechey CV, de Boer P, de Rooij DG, Evans EP, Kirk M (1983) A male-sterile insertion in the mouse. Cytogenet Cell Genet 36: 617–626.
Speed RM (1982) Meiosis in the foetal mouse ovary. I. An analysis at the light microscope level using surface-spreading. Chromosoma 85: 427–437.
Speed RM (1986) Oocyte development in XO foetuses of man and mouse: The possible role of heterologous X-chromosome pairing in germ cell survival. Chromosoma 94: 115–124.
Speed RM, Chandley AC (1983) Meiosis in the foetal mouse ovary. II. Oocyte development and age-related aneuploidy. Does a production line exist? Chromosoma 88: 184–189.
Setterfield LA, Mahadevaiah S, Mittwoch U (1988a) Chromosome pairing and germ cell loss in male and female mice carrying a reciprocal translocation. J Reprod Fertil 82: 369–379.
Setterfield LA, Mahadevaiah S, Mittwoch U (1988b) Pachytene pairing in relation to sperm and oocyte numbers in a male-fertile reciprocal translocation in the mouse. Cytogenet Cell Genet 49: 293–299.
Terasawa M, Shinohara A, Hotta Y, Ogawa H, Ogawa T (1995) Localization of RecA-like recombination proteins on chromosomes of the lily at various meiotic stages. Genes Dev 9: 925–934.
Tres LL (1977) Extensive pairing of the XY bivalent in mouse spermatocytes as visualized by whole-mount electron microscopy. J Cell Sci 25: 1–15.
Wauben-Penris PJ, van der Hoeven FA, de Boer P (1983) Chiasma frequency and nondisjunction in heteromorphic bivalents: meiotic behavior in T(1;13)70H/T(1;13)1Wa mice as compared to T(1; 13)70H/T(1;13)70H mice. Cytogenet Cell Genet 36: 547–553.
Winking H, Reuter C, Traut W (1993) Meiotic synapsis of homogeneously staining regions (HSRs) in chromosome 1 of Mus musculus. Chromosome Res 1: 37–44.
Xu Y, Ashley T, Brainerd EE, et al. (1996) Targeted disruption of ATM leads to growth retardation, chromosomal fragmentation during meiosis, immune defects, and thymic lymphoma. Genes Dev 10: 2411–2422.
Zhao GQ, Deng K, Labosky PA, Liaw L, Hogan BL (1996) The gene encoding bone morphogenetic protein 8B is required for the initiation and maintenance of spermatogenesis in the mouse. Genes Dev 10: 1657–1669.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Peters, A.H.F.M., Plug, A.W. & de Boer, P. Meiosis in Carriers of Heteromorphic Bivalents: Sex Differences and Implications for Male Fertility. Chromosome Res 5, 313–324 (1997). https://doi.org/10.1023/B:CHRO.0000038762.60086.ef
Issue Date:
DOI: https://doi.org/10.1023/B:CHRO.0000038762.60086.ef