Abstract
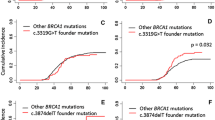
Inherited mutations of the BRCA2 gene give rise to a multi-site cancer phenotype which includes breast cancer (in female and males), ovarian, pancreatic and prostate cancer, ocular and other melanomas, laryngeal, colon and stomach cancers. Interpretation of test results and risk assessment is therefore complex. It has been proposed that families with mutations in the ovarian cancer cluster region (OCCR) of exon 11 (nucleotides 3035–6629) express a higher ratio of ovarian to breast cancer, than families with mutations elsewhere in the BRCA2 gene. In this study we have investigated the presence of 7 types of cancer (ovary, male breast, pancreas, prostate, colon, stomach and melanoma) in first- and second-degree relatives of mutation-positive individuals in 440 families with a BRCA2 mutation. We reviewed histories of cancer in relatives among families with mutations distributed throughout the gene. Families with ovarian cancer were more likely to harbour mutations in the OCCR (nucleotides 3035–6629) than elsewhere in the gene (OR = 2.21; P = 0.0002). We also compared cancer risks according to ethnic group. Ashkenazi Jewish families with the 6174delT founder mutation were more likely to have a family member with ovarian cancer (OR = 1.58; P = 0.002) and less likely to have a family member with prostate cancer (OR = 0.62; P = 0.04) than were non-Jewish families. In contrast, a reduced presence of ovarian cancer was found in families of French-Canadian ancestry, compared to other ancestries (OR = 0.37; P = 0.0026). A high risk of male breast cancer was observed with the 6503delTT mutation (OR = 15.7; P = 0.023). Families of Polish ancestry had a reduced frequency of pancreatic cancer (OR = 0.0; P = 0.03) compared to families of other ethnic origins. In conclusion, both the position of mutation and the ethnic background of the family appear to contribute to the phenotypic variation observed in families with BRCA2 mutations.
Similar content being viewed by others
References 0
Wooster R, Neuhausen S, Mangion J et al. Localisation of a breast cancer susceptibility gene (BRCA2) to chromosome 13q by genetic linkage analysis. Science 1994; 265: 2088–90. 0
Wooster R, Bignell G, Swift S et al. Identification of the breast cancer susceptibility gene BRCA2. Nature 1995; 378: 789–92. 0
Tavtigian SV, Simard J, Rommens J et al. The complete BRCA2 gene and mutations in chromosome 13q-linked kindreds. Nat Genet 1996; 12: 333–7. 0
BCLC – The Breast Cancer Linkage Consortium. Cancer risks in BRCA2 mutation carriers. J Natl Cancer Inst 1991; 91: 1310–6. 0
Risch HA, McLaughlin JR, Cole DE et al. Prevalence and penetrance of germline BRCA1 and BRCA2 mutations in a population series of 649 women with ovarian cancer. Am J Hum Genet 2001; 68: 700–10. 0
Gayther SA, Mangion J, Russell P et al. Variation of risks of breast and ovarian cancer associated with different germline mutations of the BRCA2 gene. Nat Genet 1997; 15: 103–5. 0
Thompson D, Easton D. Breast Cancer Linkage Consortium. Variation in cancer risks, by mutation position, in BRCA2 mutation carriers. Am J Hum Genet 2001; 68: 410–9. 0
Narod SA, Risch H, Moslehi R et al. Oral contraceptives and the risk of hereditary ovarian cancer. N Engl J Med 1998; 339: 424–8. 0
Narod SA, Dube M-P, Klijn J et al. Oral contraceptives and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst 2002; 94: 1773–9.
Parkin DM, Whelan SL, Ferlay J et al. Cancer Incidence in Five Continents. Lyon: IARC Press, 1997.
Jakubowska A, Nej K, Huzarski T et al. BRCA2 gene mutations in families with aggregations of breast and stomach cancers. Br J Cancer 2002; 87: 888–91.
Phelan CM, Lancaster JM, Tonin P et al. Mutation analysis of the BRCA2 gene in 49 site-specific breast cancer families. Nat Genet 1996; 13: 120–2.
Moslehi R, Chu W, Karlan B et al. BRCA1 and BRCA2 mutation analysis of 208 Ashkenazi Jewish women with ovarian cancer. Am J Hum Genet 2000; 66: 1259–72.
Sinilnikova OM, Egan KM, Quinn JL et al. Germline BRCA2 sequence variants in patients with ocular melanoma. Int J Cancer 1999; 82: 325–8.
Iscovich J, Abdulrazik M, Cour C et al. Prevalence of the BRCA2 6174delT mutation in Israeli uveal melanoma patients. Int J Cancer 2002; 98: 42–4.
Scully R, Livingston D. In search of the tumour-suppressor functions of BRCA1 and BRCA2. Nature 2000; 408: 429–32.
Venkitaraman AR. Cancer Susceptibility and the Functions of BRCA1 and BRCA2. Cell 2002; 108: 171–82.
Moynahan ME, Pierce AJ, Jasin M. BRCA2 is required for homology-directed repair of chromosomal breaks. Mol Cell 2001; 7: 263–72.
Bork P, Blomberg N, Nilges M. Internal repeats in the BRCA2 protein sequence. Nat Genet 1996; 13: 22–3.
Chen PL, Chen CF, Chen YM et al. The BRC repeats in BRCA2 are critical for RAD51 binding and resistance to methyl methanesulfonate treatment. Proc Natl Acad Sci USA 1998; 95: 5287–92.
Katagiri T, Saito H, Shinohara A et al. Multiple possible sites of BRCA2 interacting with DNA repair protein RAD51. Genes Chromosomes Cancer 1998; 21: 217–22.
Chen CF, Chen PL, Zhong Q et al. Expression of BRC repeats in breast cancer cells disrupts the BRCA2-Rad51 complex and leads to radiation hypersentsityivy and loss of G(2)/M checkpoint control. J Biol Chem 1999; 274: 32931–5.
Davies AA, Masson JY, McIlwraith MJ et al. Role of BRCA2 in control of the RAD51 recombination and DNA repair protein. Mol Cell 2001; 7: 273–82.
Xia F, Taghian DG, DeFrank JS et al. Deficiency of human BRCA2 leads to impaired homologous recombination but maintains normal nonhomologous end joining. Proc Natl Acad Sci USA 2001; 98: 8644–9.
Stark JM, Hu P, Pierce AJ et al. ATP hydrolysis by mammalian RAD51 has a key role during homology-directed DNA repair. J Biol Chem 2002; 277: 20185–94.
Yang HJ, Jeffrey PD, Miller J et al. BRCA2 function in DNA binding and recombination from a BRCA2-DSS1-ssDNA structure. Science 2002; 297: 1837–48.
Marston NJ, Richards WJ, Hughes D et al. Interaction between the product of the breast cancer susceptibility gene BRCA2 and DSS1, a protein functionally conserved from yeast to mammals. Mol Cell Biol 1999; 19: 4633–42.
Spain BH, Larson CJ, Shihabuddin LS et al. Truncated BRCA2 is cytoplasmic: Implications for cancer-linked mutations. Proc Natl Acad Sci USA 1999; 96: 13920–5.
Howlett NG, Taniguchi T, Olson S et al. Biallelic inactivation of BRCA2 in Fanconi anemia. Science 2002; 297: 606–9.
Mazoyer S, Dunning AM, Serova O et al. A polymorphic stop codon in BRCA2. Nat Genet 1996; 14: 253–4.
Zhang J, Sun XL, Qian YM et al. At least one intron is required for the nonsense-mediated decay of triosephosphate isomerase mRNA: A possible link between nuclear splicing and cytoplasmic translation. Mol Cell Biol 2002; 18: 5272–83.
Perrin-Vidoz L, Sinilnikova OM, Stoppa-Lyonet D et al. The non-sense mediated mRNA decay pathway triggers degradation of most BRCA1 mRNAs bearing premature stop codons. Hum Mol Genet 2002; 11: 2805–14.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lubinski, J., Phelan, C.M., Ghadirian, P. et al. Cancer variation associated with the position of the mutation in the BRCA2 gene. Familial Cancer 3, 1–10 (2004). https://doi.org/10.1023/B:FAME.0000026816.32400.45
Issue Date:
DOI: https://doi.org/10.1023/B:FAME.0000026816.32400.45