Abstract
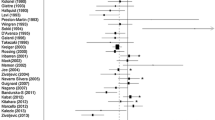
Objective: To analyze the role of smoking, alcohol, coffee and tea in relation to thyroid cancer, we conducted a pooled analysis of 14 case–control studies conducted in the United States, Europe, and Asia. Methods: The sample consisted of 2725 thyroid cancer cases (2247 females, 478 males) and 4776 controls (3699 females, 1077 males). Conditional logistic regression with stratification on study, age at diagnosis, and gender was used to compute odds ratios and 95% confidence intervals. Results: Thyroid cancer risk was reduced in persons who had ever smoked. The relationship was more pronounced in current smokers (OR = 0.6, 95% CI = 0.6–0.7) than former smokers (OR = 0.9, 95% CI = 0.8–1.1). There were significant trends of reduced risk with greater duration and frequency of smoking. For consumption of wine and beer, there was a significant trend of decreasing thyroid cancer risk (p = 0.02) that was not maintained after adjustment for current smoking (p = 0.12). Thyroid cancer risk was not associated with consumption of coffee or tea. These findings were consistent in both gender-specific and histology-specific (papillary and follicular) analyses. Conclusions: Pooled analyses of these geographically diverse case–control data indicate a reduced thyroid cancer risk associated with current smoking. A reduced risk associated with alcohol was eliminated after adjustment for smoking, and caffeinated beverages did not alter thyroid cancer risk.
Similar content being viewed by others

References
Mack WJ, Preston-Martin S (1998) Epidemiology of thyroid cancer. In: Fagin JA, ed. Thyroid Cancer. Boston: Kluwer Academic Publishers, pp. 1-25.
Galanti MR, Hansson L, Lund E, et al. (1996) Reproductive history and cigarette smoking as risk factors for thyroid cancer in women: a population-based case-control study. Cancer Epidemiol Biomarkers Prev 5: 425-431.
Rossing M, Cushing K, Voigt L, Wicklund K, Daling J (2000) Risk of papillary thyroid cancer in women in relation to smoking and alcohol consumption. Epidemiology 11: 49-54.
Kreiger N, Parkes R (2000) Cigarette smoking and the risk of thyroid cancer. Eur J Cancer 36: 1969-1973.
Williams RR (1976) Breast and thyroid cancer and malignant melanoma promoted by alcohol-induced pituitary secretion of prolactin, TSH, and MSH. Lancet I: 996-999.
Ron E, Kleinerman RA, Boice JD, LiVolsi VA, Flannery JT, Fraumeni JF (1987) A population-based case-control study of thyroid cancer. J Natl Cancer Inst 79: 1-12.
Franceschi S, Fassina A, Talamini R, et al. (1989) Risk factors for thyroid cancer in Northern Italy. Int J Epidemiol 18: 578-584.
Takezaki T, Hirose K, Inoue M, et al. (1996) Risk factors of thyroid cancer among women in Tokai, Japan. J Epidemiol 6: 140-147.
Galanti MR, Hansson L, Bergstrom R, et al. (1997) Diet and the risk of papillary and follicular thyroid carcinoma: a populationbased case-control study in Sweden and Norway. Cancer Causes Control 8: 205-214.
Linos A, Linos DA, Vgotza N, Souvatzoglou A, Koutras DA (1989) Does coffee consumption protect against thyroid disease? Acta Chir Scand 155: 317-320.
Franceschi S, Levi F, Negri E, Fassina A, LaVecchia C (1991) Diet and thyroid cancer: a pooled analysis of four European case-control studies. Int J Cancer 48: 395-398.
Franceschi S, Preston-Martin S, Dal Maso L, et al. (1999) A pooled analysis of case-control studies of thyroid cancer. IV. Benign thyroid diseases. Cancer Causes Control 10: 583-595.
Dal Maso L, La Vecchia C, Franceschi S, et al. (2000) A pooled analysis of thyroid cancer studies. V. Anthropometric factors. Cancer Causes Control 11: 137-144.
Negri E, Ron E, Franceschi S, et al. (1999) A pooled analysis of case-control studies of thyroid cancer. I. Methods. Cancer Causes Control 10: 131-142.
McTiernan AM, Weiss NS, Daling JR (1984) Incidence of thyroid cancer in women in relation to reproductive and hormonal factors. Am J Epidemiol 120: 423-435.
Preston-Martin S, Bernstein L, Pike MC, Maldonado AA, Henderson BE (1987) Thyroid cancer among young women related to prior thyroid disease and pregnancy history. Br J Cancer 55: 191-195.
Kolonel LN, Hankin JH, Wilkens LR, Fukunaga FH, Hinds MW (1990) An epidemiologic study of thyroid cancer in Hawaii. Cancer Causes Control 1: 223-234.
Preston-Martin S, Jin F, Duda MJ, Mack WJ (1993) A case-control study of thyroid cancer in women under age 55 in Shanghai (People's Republic of China). Cancer Causes Control 4: 431-440.
Wingren G, Hatschek T, Axelson O (1993) Determinants of papillary cancer of the thyroid. Am J Epidemiol 138: 482-491.
Hallquist A, Hardell L, Degerman A, Boquist L (1993) Occupational exposures and thyroid cancer: results of a case-control study. Eur J Cancer Prev 2: 345-349.
Glattre E, Haldorsen T, Berg JP, Stensvold I, Solvoll K (1993) Norwegian case-control study testing the hypothesis that seafood increases the risk of thyroid cancer. Cancer Causes Control 4: 11-16.
D'Avanzo B, LaVecchia C, Franceschi S, Negri E, Talamini R (1995) History of thyroid diseases and subsequent thyroid cancer risk. Cancer Epidemiol Biomarkers Prev 4: 193-199.
Levi F, Franceschi S, Gulie C, Negri E, LaVecchia C (1993) Female thyroid cancer: the role of reproductive and hormonal factors in Switzerland. Oncology 50: 309-315.
Henderson BE, Ross RK, Pike MC, Casagrande JT (1982) Endogenous hormones as a major factor in human cancer. Cancer Res 42: 3232-3239.
Williams ED (1990) TSH and thyroid cancer. Horm Metab Res 23: 72-75.
Eden S, Jagenburg R, Lindstedt G, Lundberg PA, Mellstrom D (1984) Thyroregulatory changes associated with smoking in 70-year-old men. Clin Endocrinol 21: 605-610.
Christensen SB, Ericsson UB, Janzon L, Tibblin S, Melander A (1984) Influence of cigarette smoking on goiter formation, thyroglobulin, and thyroid hormone levels in women. J Clin Endocrinol Metab 58: 615-618.
Ericsson UB, Lindgarde F (1991) Effects of cigarette smoking on thyroid function and the prevalence of goitre, thyrotoxicosis and autoimmune thyroiditis. J Int Med 229: 67-71.
Fisher CL, Mannino DM, Herman WH, Frumkin H (1997) Cigarette smoking and thyroid hormone levels in males. Int J Epidemiol 26: 972-977.
Karakaya A, Tuncel N, Alptuna G, Kocer Z, Erbay G (1987) Influence of cigarette smoking on thyroid hormone levels. Human Toxicol 6: 507-509.
Melander A, Nordenskjold E, Lundh B, Thorell J (1981) Influence of smoking on thyroid activity. Acta Medica Scandanavia 209: 41-43.
Hegedus L, Karstrup S, Veiergang D, Jacobsen B, Skovsted L, Feldt-Rasmussen U (1985) High frequency of goitre in cigarette smokers. Clin Endocrinol 22: 287-292.
Fukayama H, Nasu M, Murakami S, Sugawara M (1992) Examination of antithyroid effects of smoking products in cultured thyroid follicles: only thiocyanate is a potent antithyroid agent. Acta Endocrinol 127: 520-525.
Baron JA, LaVecchia C, Levi F (1990) The antiestrogenic effect of cigarette smoking in women. Am J Obstet Gynecol 162: 502-514.
Grady D, Ernster V (1996) Endometrial Cancer. In: Schottenfeld D, Fraumeni J, eds. Cancer Epidemiology and Prevention. New York: Oxford University Press, pp. 1058-1089.
Key T, Pike M, Brown J, Hermon C, Allen D, Wang D (1996) Cigarette smoking and urinary oestrogen excretion in premenopausal and post-menopausal women. Br J Cancer 74: 1313-1316.
Thomas E, Edridge W, Weddell A, McGill A, McGarrigle H (1993) The impact of cigarette smoking on the plasma concentrations of gonadotrophins, ovarian steroids and androgens and upon the metabolism of oestrogens in the human female. Human Reprod 8: 1187-1193.
Berta L, Fortunati N, Gennari P, Appendino M, Casella A, Frairia R (1991) Influence of cigarette smoking on pituitary and sex hormone balance in healthy premenopausal women. Fertil Steril 56: 788-789.
Cassidenti DL, Pike MC, Vijod AG, Stanczyk FZ, Lobo RA (1992) A reevaluation of estrogen status in postmenopausal women who smoke. Am J Obstet Gynecol 166: 1444-1448.
Longcope C, Johnston C (1988) Androgen and estrogen dynamics in pre-and postmenopausal women: a comparison between smokers and nonsmokers. J Clin Endocrinol Metab 67: 379-383.
Dai WS, Gutai JP, Kuller LH, Cauley JA (1988) Cigarette smoking and serum sex hormones in men. Am J Epidemiol 128: 796-805.
Cassidenti DL, Vijod AG, Vijod MA, Stanczyk FZ, Lobo RA (1990) Short-term effects of smoking on the pharmocokinetic profiles of micronized estradiol in postmenopausal women. Am J Obstet Gynecol 163: 1953-1960.
Michnovicz J, Hershcopf R, Haley N, Bradlow H, Fishman J (1989) Cigarette smoking alters hepatic estrogen metabolism in men: implications for atherosclerosis. Metab Clin Exp 38: 537-541.
Michnovicz J, Hershcopf R, Naganuma H, Bradlow H, Fishman J (1986) Increased 2-hydroxylation of estradiol as a possible mechanism for the anti-estrogenic effect of cigarette smoking. N Engl J Med 315: 1305-1309.
Law M, Cheng R, Hackshaw A, Allaway S, Hale A (1997) Cigarette smoking, sex hormones and bone density in women. Eur J Epidemiol 13: 553-558.
Ron E (1996) Thyroid Cancer. In: Schottenfeld D, Fraumeni JF, eds. Cancer Epidemiology and Prevention. New York: Oxford University Press, pp. 1000-1021.
Knudsen N, Bulow I, Laurberg P, Perrild H, Ovesen L, Jorgensen T (2001) Alcohol consumption is associated with reduced prevalence of goitre and solitary thyroid nodules. Clin Endocrinol 55: 41-46.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mack, W.J., Preston-Martin, S., Dal Maso, L. et al. A pooled analysis of case–control studies of thyroid cancer: cigarette smoking and consumption of alcohol, coffee, and tea. Cancer Causes Control 14, 773–785 (2003). https://doi.org/10.1023/A:1026349702909
Issue Date:
DOI: https://doi.org/10.1023/A:1026349702909