Abstract
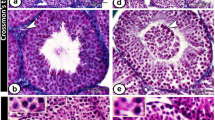
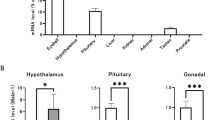
We have generated and analysed null mutations in the mouse genes encoding three structurally related receptors with tyrosine kinase activity: Tyro 3, Axl, and Mer1,4. Mice lacking any single receptor, or any combination of two receptors, are viable and fertile, but male animals that lack all three receptors produce no mature sperm, owing to the progressive death of differentiating germ cells. This degenerative phenotype appears to result from a failure of the tropic support that is normally provided by Sertoli cells of the seminiferous tubules, whose function depends on testosterone and additional factors produced by Leydig cells5,7. Tyro 3, Axl and Mer are all normally expressed by Sertoli cells during postnatal development, whereas their ligands, Gas6 and protein S, are produced by Leydig cells before sexual maturity, and by both Leydig and Sertoli cells thereafter. Here we show that the concerted activation of Tyro 3, Axl and Mer in Sertoli cells is critical to the role that these cells play as nurturers of developing germ cells. Additional observations indicate that these receptors may also be essential for the tropic maintenance of diverse cell types in the mature nervous, immune and reproductive systems.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Crosier, K. E. & Crosier, P. S. New insights into the control of cell growth; the role of the Axl family. Pathology 29, 131–135 (1997).
Lai, C., Gore, M. & Lemke, G. Structure, expression, and activity of Tyro 3, a neural adhesion-related receptor tyrosine kinase. Oncogene 9, 2567–2578 (1994).
O'Bryan, J. P. et al. Axl, a transforming gene isolated from primary human myeloid leukemia cells, encodes a novel receptor tyrosine kinase. Mol. Cell. Biol. 11, 5016–5031 (1991).
Graham, D. K., Dawson, T. L., Mullaney, D. L., Snodgrass, H. R. & Earp, H. S. Cloning and mRNA expression analysis of a novel human protooncogene, c-mer. Cell Growth Differ. 5, 647–657 (1994).
Skinner, M. K. Cell-cell interactions in the testis. Endocrine Rev. 12, 45–77 (1991).
Skinner, M. K. in The Sertoli Cell (eds Russell, L. D. & Griswold, M. D.) 237–247 (Cache River, Clearwater, Florida, 1993).
Griswold, M. D. Interactions between germ cells and Sertoli cells in the testis. Biol. Reprod. 52, 211–216 (1995).
Lai, C. & Lemke, G. An extended family of protein-tyrosine kinase genes differentially expressed in the vertebrate nervous system. Neuron 6, 691–704 (1991).
Mark, M. R. et al. RSE, a novel receptor-type tyrosine kinase with homology to Axl/Ufo, is expressed at high levels in the brain. J. Biol. Chem. 269, 10720–10728 (1994).
Taylor, I. C., Roy, S., Yaswen, P., Stampfer, M. R. & Varmus, H. E. Mouse mammary tumors express elevated levels of RNA encoding the murine homology of SKY, a putative receptor tyrosine kinase. J. Biol. Chem. 270, 6872–6880 (1995).
Rescigno, J., Mansukhani, A. & Basilico, C. Aputative receptor tyrosine kinase with unique structural topology. Oncogene 6, 1909–1913 (1991).
Janssen, J. W. et al. Anovel putative tyrosine kinase receptor with oncogenic potential. Oncogene 6, 2113–2120 (1991).
Jia, R. & Hanafusa, H. The proto-oncogene of v-eyk (v-ryk) is a novel receptor-type protein tyrosine kianse with extracellular Ig/GN-III domains. J. Biol. chem. 269, 1839–1844 (1994).
Stitt, T. N. et al. The anticoagulation factor protein S and its relative, Gas6, are ligands for the Tyro 3/Axl family of receptor tyrosine kinases. Cell 80, 661–670 (1995).
Godowski, P. J. et al. Reevaluation of the roles of protein S and Gas6 as ligands for the receptor tyrosine kinase Rse/Tyro 3. Cell 82, 355–358 (1995).
Chen, J., Carey, K. & Godowski, P. J. Identification of Gas6 as a ligand for Mer, a neural cell adhesion molecule related receptor tyrosine kinase implicated in cellular transformation. Oncogene 14, 2033–2039 (1997).
Nagata, K. et al. Identification of the product of growth arrest-specific gene 6 as a common ligand for Axl, Sky, and Mer receptor tyrosine kinases. J. Biol. Chem. 271, 30022–30027 (1996).
Joseph, D. R. Sequence and functional relationships between androgen-binding protein/sex hormone-binding globulin and its homologs protein S, Gas6, laminin, and agrin. Steroids 62, 578–588 (1997).
Capecchi, M. R. Altering the genome by homologous recombination. Science 244, 1288–1292 (1989).
Camenisch, T. D., Koller, B. H., Earp, H. S. & Matsushima, G. K. Anovel receptor tyrosine kinase, Mer, inhibits TNF-α production and lipopolysaccaride-induced endotoxic shock. J. Immunol. 162, 3498–3503 1999).
Smiley, S. T., Stitt, T. N. & Grusby, M. J. Cross-linking of protein S bound to lymphocytes promotes aggregation and inhibits proliferation. Cell. Immunol. 181, 120–126 (1997).
Leblond, C. P. & Clemont, Y. Definition of the stages of the cycle of the seminiferous epithelium in the rat. Ann. N. Y. Acad. Sci. 55, 548–573 (1952).
Henriksén, K., Hakovirta, H. & Parvinen, M. Testosterone inhibits and induces apoptosis in rat seminiferous tubules in a stage-specific manner. Endocrinology 136, 3285–3291 (1995).
Sun, Y.-, Wreford, N. G., Robertson, D. M. & De Kretser, D. M. Quantitative cytological studies of spermatogenesis in intact and hypophysectomized rats: Identification of androgen-dependent stages. Endocrinology 127, 1215–1223 (1991).
Wong, V. & Russell, L. D. Three-dimensional reconstruction of a rat stage V Sertoli cell: I. Methods, basic configuration, and dimensions. Am. J. Anat. 167, 143–161 (1983).
Yomogida, K. et al. Developmental stage- and spermatogenic cycle-specific expression of transcription factor GATA-1 in mouse Sertoli cells. Development 120, 1759–1766 (1994).
Lewis, P. A., Crosier, K. E., Wood, C. R. & Crosier, P. S. Analysis of the murine Dtk gene identifies conservation of genomic structure within a new receptor tyrosine kinase subfamily. Genomics 31, 13–19 (1996).
Biesecker, L. G., Giannola, D. M. & Emerson, S. G. Identification of alternative exons, including a novel exon, in the tyrosine kinase receptor gene Etk2/tyro3 that explains differences in 5′ cDNA sequences. Oncogene 10, 2239–2242 (1995).
Schulz, A. S., Schleithoff, L., Faust, M., Bartram, C. R. & Janssen, J. W. G. The genomic structure of the human UFO receptor. Oncogene 8, 509–513 (1993).
Bellosta, P., Zhang, Q., Goff, S. P. & Basilico, C. Signaling through the ARK tyrosine kinase receptor protects from apoptosis in the absence of growth stimulation. Oncogene 15, 2387–2397 (1997).
Acknowledgements
This work was supported by grants from the NIH (to G.L., S.P.G., H.S.E., G.K.M.), and by a Markey fellowship to M.G., who was a student in the Neurosciences graduate program at the University of California, San Diego. S.P.G. is an Investigator of the Howard Hughes Medical Institute. We thank A. Prieto for advice and for Gas6 antibody, G. Yancopoulos for Gas6, protein S, and Axl cDNA probes, and D. Ortuño, P. Burrola, and D. Baynes for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, Q., Gore, M., Zhang, Q. et al. Tyro-3 family receptors are essential regulators of mammalian spermatogenesis. Nature 398, 723–728 (1999). https://doi.org/10.1038/19554
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/19554
This article is cited by
-
Receptor tyrosine kinases Tyro3, Axl, and Mertk differentially contribute to antibody-induced arthritis
Cell Communication and Signaling (2023)
-
Phagocytosis in the retina promotes local insulin production in the eye
Nature Metabolism (2023)
-
Regulation of brain endothelial cell physiology by the TAM receptor tyrosine kinase Mer
Communications Biology (2023)
-
Therapeutic targeting of the functionally elusive TAM receptor family
Nature Reviews Drug Discovery (2023)
-
Axl receptor induces efferocytosis, dampens M1 macrophage responses and promotes heart pathology in Trypanosoma cruzi infection
Communications Biology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.