Abstract
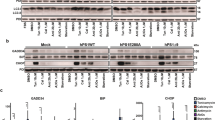
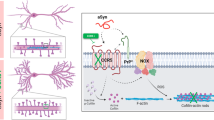
Mutations of the presenilin-1 gene are a major cause of familial early-onset Alzheimer's disease1,2,3,4. Presenilin-1 can associate with members of the catenin family of signalling proteins, but the significance of this association is unknown5,6. Here we show that presenilin-1 forms a complex with β-catenin in vivo that increases β-catenin stability. Pathogenic mutations in the presenilin-1 gene reduce the ability of presenilin-1 to stabilize β-catenin, and lead to increased degradation of β-catenin in the brains of transgenic mice. Moreover, β-catenin levels are markedly reduced in the brains of Alzheimer's disease patients with presenilin-1 mutations. Loss of β-catenin signalling increases neuronal vulnerability to apoptosis induced by amyloid-β protein. Thus, mutations in presenilin-1 may increase neuronal apoptosis by altering the stability of β-catenin, predisposing individuals to early-onset Alzheimer's disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Sherrington, R. et al . Cloning of a gene bearing missense mutations in early-onset familial Alzheimer's disease. Nature 375, 754–760 (1995).
Levy-Lahad, E. et al . Candidate gene for chromosome 1 familial Alzheimer's disease locus. Science 269, 973–977 (1995).
Rogaev, E. I. et al . Familial Alzheimer's disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer's disease type 3 gene. Nature 376, 775–778 (1995).
Li, J., Ma, J. & Potter H. Identification and expression of a potential familial Alzheimer disease gene on chromosome 1 related to AD3. Proc. Natl Acad. Sci. USA 92, 12180–12184 (1995).
Zhou, J. et al . Presenilin 1 interaction in the brain with a novel member of the Armadillo family. Neuroreport 8, 1489–1494 (1997).
Yu, G. et al . The presenilin 1 protein is a component of a high molecular weight complex that contains β-catenin. J. Biol. Chem. 273, 164–16475 (1998).
Busciglio, J. et al . Neuronal localization of presenilin-1 and association with amyloid plaques and neurofibrillary tangles in Alzheimer's disease. J. Neurosci. 17, 5101–5107 (1997).
Thinakaran, G. et al . Endoproteolysis of presenilin 1 and accumulation of processed derivatives in vivo. Neuron 17, 181–190 (1996).
Nusse, R. Aversatile transcription effector of wingless signalling. Cell 89, 321–323 (1997).
Kovacs, D. M. et al . Alzheimer-associated presenilins 1 and 2: neuronal expression in brain and localization to intracellular membranes in mammalian cells. Nature Med. 2, 224–229 (1996).
Walter, J. et al . Alzheimer's disease-associated presenilins are differentially phosphorylated proteins located within the endoplasmic reticulum. Mol. Med. 2, 673–691 (1996).
De Strooper, B. et al . Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature 391, 387–390 (1998).
Yankner, B. A. Mechanisms of neuronal degeneration in Alzheimer's disease. Neuron 16, 921–932 (1996).
Molenaar, M. et al . Xtcf-3 transcription factor mediates β-catenin-induced axis formation in Xenopus embryos. Cell 86, 391–399 (1996).
van de Wetering, M. et al . Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell 88, 789–799 (1997).
Capell, A. et al . The proteolytic fragments of the Alzheimer's disease-associated presenilin-1 form heterodimers and occur as a 100–150-kDa molecular mass complex. J. Biol. Chem. 273, 3205–3211 (1998).
Scheuner, D. et al . Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer's disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer's disease. Nature Med. 2, 864–870 (1996).
Duff, K. et al . Increased amyloid-β42(43) in brains of mice expressing presenilin 1. Nature 383, 710–713 (1996).
Borchelt, D. R. et al . Familial Alzheimer's disease-linked presenilin-1 variants elevate Aβ1-42/1-40 ratio in vitro and in vivo. Neuron 17, 1005–1013 (1996).
Citron, M. et al . Mutant presenilins of Alzheimer's disease increase production of 42-residue amyloid beta-protein in both transfected cells and transgenic mice. Nature Med. 3, 67–72 (1997).
Morin, P. J., Vogelstein, B. & Kinzler, K. W. Apoptosis and APC in colorectal tumorigenesis. Proc. Natl Acad. Sci. USA 93, 7950–7954 (1996).
Ahmed, Y., Hayashi, S., Levine, A. & Wieschaus, E. Regulation of Armadillo by a Drosophila APC inhibits neuronal apoptosis during retinal development. Cell 93, 1171–1182 (1998).
Wolozin, B. et al . Participation of presenilin 2 in apoptosis: enhanced basal activity conferred by an Alzheimer mutation. Science 274, 1710–1713 (1996).
Guo, Q. et al . Alzheimer's presenilin mutation sensitizes neural cells to apoptosis induced by trophic factor withdrawal and amyloid β-peptide: involvement of calcium and oxyradicals. J. Neurosci. 17, 4212–4222 (1997).
Guo, Q. et al . Par-4 is a mediator of neuronal degeneration associated with the pathogenesis of Alzheimer disease. Nature Med. 4, 957–962 (1998).
Sturchler-Pierrat, C. et al . Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc. Natl Acad. Sci. USA 94, 13287–13292 (1997).
Dudek, H. et al . Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science 275, 661–665 (1997).
Lorenzo, A. & Yankner, B. A. Beta-amyloid neurotoxicity requires fibril formation and is inhibited by Congo Res. Proc. Natl Acad. Sci. USA 91, 12243–12247 (1994).
Acknowledgements
We thank D. Nochlin, C. Lippa, T. Bird, C. Rosenberg, A. Roses, D. Pollin and J. Rogers for autopsy human brain tissue; K. Burki and B. Lederman for assistance in the generation of transgenic mice; and Y. Sun for discussions. This work was supported by grants from the NIH, the Alzheimer's Association and Novartis Pharma Ltd (to B.A.Y.), an NIH training grant and a fellowship from The Medical Foundation (to Z.Z.), a fellowship from the Deutsche Forschungsgemeinschaft (to H.H.), a Pew Scholarship (to X.H.), and an NIH MRRC Core Grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Z., Hartmann, H., Minh Do, V. et al. Destabilization of β-catenin by mutations in presenilin-1 potentiates neuronal apoptosis. Nature 395, 698–702 (1998). https://doi.org/10.1038/27208
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/27208
This article is cited by
-
Telomerase reverse transcriptase preserves neuron survival and cognition in Alzheimer’s disease models
Nature Aging (2021)
-
Impaired Wnt Signaling in the Prefrontal Cortex of Alzheimer’s Disease
Molecular Neurobiology (2019)
-
Guanosine monophosphate reductase 1 is a potential therapeutic target for Alzheimer’s disease
Scientific Reports (2018)
-
Role of Wnt Signaling in Central Nervous System Injury
Molecular Neurobiology (2016)
-
The Role of Presenilin in Protein Trafficking and Degradation—Implications for Metal Homeostasis
Journal of Molecular Neuroscience (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.