Abstract
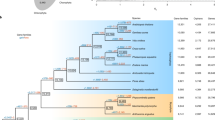
The first evidence for the emergence of land plants (embryophytes) consists of mid-Ordovician spore tetrads (∼476 Myr old)1,2. The identity of the early plants that produced these spores is unclear; they are sometimes claimed to be liverworts3,4, but there are no associated megafossils, and similar spores can be produced by a diversity of plants2. Indeed, the earliest unequivocal megafossils of land plants consist of early vascular plants and various plants of uncertain affinity1. Different phylogenetic analyses have identified liverworts, hornworts and bryophytes as each being the first lineage of land plants1,2,5,6,7,8,9,10,11,12,13; the consensus of these conflicting topologies yields an unresolved polychotomy at the base of land plants. Here we survey 352 diverse land plants and find that three mitochondrial group II introns are present, with occasional losses, in mosses, hornworts and all major lineages of vascular plants, but are entirely absent from liverworts, green algae and all other eukaryotes. These results indicate that liverworts are the earliest land plants, with the three introns having been acquired in a common ancestor of all other land plants, and have important implications concerning the early stages of plant evolution.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Kenrick, P. & Crane, P. R. The origin and early evolution of plants on land. Nature 389, 33–39 (1997).
Gray, J. Major paleozoic land plant evolutionary bio-events. Palaeogeogr. Palaeoclimatol. Palaeoecol. 104, 153–169 (1993).
Edwards, D., Duckett, J. G. & Richardson, J. B. Hepatic characters in the earliest land plants. Nature 374, 635–636 (1995).
Taylor, W. A. Spores in earliest land plants. Nature 373, 391–392 (1995).
Mishler, B. D. et al. Phylogenetic relationships of the “green algae” and “bryophytes”. Ann. Mo. Bot. Gard. 81, 451–483 (1994).
Kenrick, P. & Crane, P. R. The Origin and Early Diversification of Land Plants: a Cladistic Study (Smithsonian Institution Press, Washington DC, (1997)).
Lewis, L. A., Mishler, B. D. & Vilgalys, R. Phylogenetic relationships of the liverworts (Hepaticae), a basal embryophyte lineage inferred from nucleotide sequence data of the chloroplast gene rbcL . Mol. Phylogen. Evol. 7, 377–393 (1997).
Hedderson, T. A., Chapman, R. L. & Rootes, W. L. Phylogenetic relationships of bryophytes inferred from nuclear-encoded rRNA gene sequences. Plant Syst. Evol. 200, 213–224 (1996).
Malek, L., Lättig, K., Hiesel, R., Brennicke, A. & Knoop, V. RNA editing in bryophytes and a molecular phylogeny of land plants. EMBO J. 15, 1403–1411 (1996).
Graham, L. E. Origin of Land Plants (John Wiley, New York,(1993)).
Garbary, D. J., Renzaglia, K. S. & Duckett, J. G. The phylogeny of land plants: a cladistic analysis based on male gametogenesis. Plant Syst. Evol. 188, 237–269 (1993).
Manhart, J. R. Phylogenetic analysis of green plant rbcL sequences. Mol. Phylogen. Evol. 3, 114–127 (1994).
Capesius, I. & Bopp, M. New classification of liverworts based on molecular and morphological data. Plant. Syst. Evol. 207, 87–97 (1997).
Oda, K. et al. Gene organization deduced from the complete sequence of liverwort Marchantia polymorpha mitochondrial DNA: a primitive form of plant mitochondrial genome. J. Mol. Biol. 223, 1–7 (1992).
Nugent, J. M. & Palmer, J. D. in Plant Mitochondria (eds Brennicke, A. & Kück, U.), 163–170 (VCH, Weinheim, (1993)).
Wolfe, K. H., Li, W.-H. & Sharp, P. M. Rates of nucleotide substitution vary greatly among plant mitochondrial, chloroplast, and nuclear DNAs. Proc. Natl Acad. Sci. USA 84, 9054–9058 (1987).
Palmer, J. D. & Herbon, L. A. Plant mitochondrial DNA evolves rapidly in structure, but slowly in sequence. J. Mol. Evol. 28, 87–97 (1988).
Samigullin, T. H. et al. Sequences of rDNA internal transcribed spacers from the chloroplast DNA of 26 bryophytes: properties and phylogenetic utilities. FEBS Lett. 422, 47–51 (1998).
Sluiman, H. J. Acladistic evaluation of the lower and higher green plants. Plant Syst. Evol. 149, 217–232 (1985).
Doyle, J. J. & Doyle, J. S. Arapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 19, 11–15 (1987).
Doyle, J. A. & Donoghue, M. J. Seed plant phylogeny and the origin of angiosperms: an experimental cladistic approach. Bot. Rev. 52, 321–431 (1986).
Pryer, K. M., Smith, A. R. & Skog, J. E. Phylogenetic relationships of extant ferns based on evidence from morphology and rbcL sequences. Am. Fern J. 85, 205–282 (1995).
Chaw, S.-M., Zharkikh, A., Sung, H.-M., Lau, T.-C. & Li, W.-H. Molecular phylogeny of extant gymnosperms and seed plant evolution: analysis of nuclear 18S rRNA sequences. Mol. Biol. Evol. 14, 56–68 (1997).
Acknowledgements
We thank K. Adams, G. J. Gastony and P. Kuhlman for critical reading of the manuscript, R. C. Brown, B. Crandall-Stotler, G. J. Gastony, B. D. Mishler, K. S. Renzaglia, J. Shaw, H.J.Sluiman, A. R. Smith, S. H. Strauss, D. Waters and J. A. Wheeler for plant material, F. Dong and K.G.Wilson for probes, and G. Burger, M. W. Gray, B. F. Lang, C. Lemieux and M. Turmel for unpublished data. This work was supported by grants to Y.Q. and J.D.P. from the NIH.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
Supplementary Information (PDF 729 kb)
Rights and permissions
About this article
Cite this article
Qiu, YL., Cho, Y., Cox, J. et al. The gain of three mitochondrial introns identifies liverworts as the earliest land plants. Nature 394, 671–674 (1998). https://doi.org/10.1038/29286
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/29286
This article is cited by
-
Categorizing 161 plant (streptophyte) mitochondrial group II introns into 29 families of related paralogues finds only limited links between intron mobility and intron-borne maturases
BMC Ecology and Evolution (2023)
-
Genome-wide characterization and expression analysis of Erf gene family in cotton
BMC Plant Biology (2022)
-
Genome-wide identification and expression analysis of AP2/ERF transcription factors in sugarcane (Saccharum spontaneum L.)
BMC Genomics (2020)
-
An ancient tropical origin, dispersals via land bridges and Miocene diversification explain the subcosmopolitan disjunctions of the liverwort genus Lejeunea
Scientific Reports (2020)
-
Demystifying the liverwort Radula marginata, a critical review on its taxonomy, genetics, cannabinoid phytochemistry and pharmacology
Phytochemistry Reviews (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.