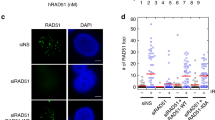
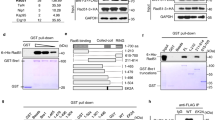
Abstract
The generation of a double-strand break in the Saccharomyces cerevisiae genome is a potentially catastrophic event that can induce cell-cycle arrest or ultimately result in loss of cell viability.The repair of such lesions is strongly dependent on proteins encoded by the RAD52 epistasis group of genes (RAD50-55, RAD57, MRE11, XRS2)1,2, as well as the RFA13,4 and RAD59 genes5. rad52 mutants exhibit the most severe phenotypic defects in double-strand break repair2, but almost nothing is known about the biochemical role of Rad52 protein. Rad51 protein promotes DNA strand exchange6,7,8 and acts similarly to RecA protein9. Yeast Rad52 protein interacts with Rad51 protein10,11, binds single-stranded DNA and stimulates annealing of complementary single-stranded DNA12. We find that Rad52 protein stimulates DNA strand exchange by targeting Rad51 protein to a complex of replication protein A (RPA) with single-stranded DNA. Rad52 protein affects an early step in the reaction, presynaptic filament formation, by overcoming the inhibitory effects of the competitor, RPA. Furthermore, stimulation is dependent on the concerted action of both Rad51 protein and RPA, implying that specific protein–protein interactions between Rad52 protein, Rad51 protein and RPA are required.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Game, J. C. DNA double-strand breaks and the RAD50–RAD57 genes in Saccharomyces . Semin. Cancer Biol. 4, 73– 83 (1993).
Petes, T. D., Malone, R. E. & Symington, L. S. in The Molecular and Cellular Biology of the Yeast Saccharomyces: Genome Dynamics, Protein Synthesis, and Energetics (eds Broach, J. R., Jones, E. & Pringle, J.) 407 (Cold Spring Harbor Laboratory, New York, 1991).
Smith, J. & Rothstein, R. Amutation in the gene encoding the Saccharomyces cerevisiae single-stranded DNA-binding protein Rfa1 stimulates a RAD52-independent pathway for direct-repeat recombination. Mol. Cell. Biol. 15, 1632–1641 (1995).
Firmenich, A. A., Elias-Arnanz, M. & Berg, P. Anovel allele of Saccharomyces cerevisiae RFA1 that is deficient in recombination and repair and suppressible by RAD52. Mol. Cell. Biol. 15, 1620–1631 (1995).
Bai, Y. & Symington, L. S. ARad52 homolog is required for RAD51-independent mitotic recombination in Saccharomyces cerevisiae. Genes Dev. 10, 2025–2037 (1996).
Sung, P. Catalysis of ATP-dependent homologous DNA pairing and strand exchange by yeast RAD51 protein. Science 265, 1241– 1243 (1994).
Sung, P. & Robberson, D. L. DNA strand exchange mediated by a RAD51-ssDNA nucleoprotein filament with polarity opposite to that of RecA. Cell 82, 453–461 (1995).
Sung, P. & Stratton, S. A. Yeast Rad51 recombinase mediates polar DNA strand exchange in the absence of ATP hydrolysis. J. Biol. Chem. 271, 27983–27986 (1996).
Kowalczykowski, S. C. & Eggleston, A. K. Homologous pairing and DNA strand-exchange proteins. Annu. Rev. Biochem. 63, 991–1043 (1994).
Shinohara, A., Ogawa, H. & Ogawa, T. Rad51 protein involved in repair and recombination in S. cerevisiae is a RecA-like protein. Cell 69, 457–470 (1992). [Erratum, Cell 71, 180; (1992 )].
Milne, G. T. & Weaver, D. T. Dominant negative alleles of RAD52 reveal a DNA repair/recombination complex including Rad51 and Rad52. Genes Dev. 7, 1755–1765 (1993).
Mortensen, U. H., Bendixen, C., Sunjevaric, I. & Rothstein, R. DNA strand annealing is promoted by the yeast Rad52 protein. Proc. Natl Acad. Sci. USA 93, 10729–10734 (1996).
Kowalczykowski, S. C., Dixon, D. A., Eggleston, A. K., Lauder, S. D. & Rehrauer, W. M. Biochemistry of homologous recombination in Escherichia coli. Microbiol. Rev. 58, 401–465 (1994).
Wold, M. S. Replication protein A: a heterotrimeric, single-stranded DNA-bindign protein required for eukaryotic DNA metabolism. Annu. Rev. Biochem. 66, 61–92 (1997).
Sugiyama, T., Zaitseva, E. M. & Kowalczykowski, S. C. Asingle-stranded DNA-binding protein is needed for efficient presynaptic complex formation by the Saccharomyces cerevisiae Rad51 protein. J. Biol. Chem. 272, 7940–7945 (1997).
Sung, P. Yeast Rad55 and Rad57 proteins form a heterodimer that functions with replication protein A to promote DNA strand exchange by Rad51 recombinase. Genes Dev. 11, 1111–1121 (1997).
Sung, P. Function of yeast Rad52 protein as mediator between replication protein A and the Rad51 recombinase. J. Biol. Chem. 272, 28194–28197 (1997).
Park, M. S., Ludwig, D. L., Stigger, E. & Lee, S. H. Physical interaction between human RAD52 and RPA is required for homologous recombination in mammalian cells. J. Biol. Chem. 271 , 18996–19000 (1996).
Yonesaki, T. & Minagawa, T. Synergistic action of three recombination gene products of bacteriophage T4, uvsX, uvsY, and gene 32 proteins. J. Biol. Chem. 264, 7814–7820 (1989).
Jiang, H., Giedroc, D. & Kodadek, T. The role of protein–protein interactions in the assembly of the presynaptic filament for T4 homologous recombination. J. Biol. Chem. 268, 7904–7911 (1993).
Sugawara, N.et al. DNA structure-dependent requirements for yeast RAD genes in gene conversion. Nature 373, 84– 86 (1995).
Rattray, A. J. & Symington, L. S. Use of a chromosomal inverted repeat to demonstrate that the RAD51 and RAD52 genes of Saccharomyces cerevisiae have different roles in mitotic recombination. Genetics 138, 587–595 (1994).
Kowalczykowski, S. C. & Krupp, R. A. Effects of the Escherichia coli SSB protein on the single-stranded DNA-dependent ATPase activity of Escherichia coli RecA protein: Evidence that SSB protein facilitates the binding of RecA protein to regions of secondary structure within single-stranded DNA. J. Mol. Biol. 193, 97– 113 (1987).
Acknowledgements
We thank T. Ogawa for antibodies against Rad52 and Rad51 proteins, K. Adzuma for advice on the purification of Rad52 protein, E. Zaitsev for strains, and T. Ogawa, A. Shinohara, P. Sung and S. West for communication of unpublished results. This work was supported by grants from the NIH and from the Human Frontier Science Program (to S.C.K.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
New, J., Sugiyama, T., Zaitseva, E. et al. Rad52 protein stimulates DNA strand exchange by Rad51 and replication protein A. Nature 391, 407–410 (1998). https://doi.org/10.1038/34950
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/34950
This article is cited by
-
Bre1/RNF20 promotes Rad51-mediated strand exchange and antagonizes the Srs2/FBH1 helicases
Nature Communications (2023)
-
ssDNA accessibility of Rad51 is regulated by orchestrating multiple RPA dynamics
Nature Communications (2023)
-
Rtt105 regulates RPA function by configurationally stapling the flexible domains
Nature Communications (2022)
-
Nucleotide proofreading functions by nematode RAD51 paralogs facilitate optimal RAD51 filament function
Nature Communications (2021)
-
Alternative lengthening of telomeres: from molecular mechanisms to therapeutic outlooks
Cell & Bioscience (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.