Abstract
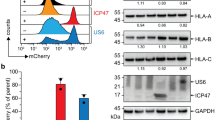
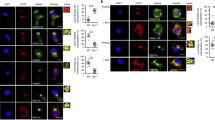
Major-histocompatibility-complex (MHC) proteins are used to display, on the surface of a cell, peptides derived from foreign material — such as a virus — that is infecting that cell. Cytotoxic T lymphocytes then recognize and kill the infected cell. The HIV-1 Nef protein downregulates the cell-surface expression of class I MHC proteins, and probably thereby promotes immune evasion by HIV-1. In the presence of Nef, class I MHC molecules are relocalized from the cell surface to the trans-Golgi network (TGN) through as-yet-unknown mechanisms. Here we show that Nef-induced downregulation of MHC-I expression and MHC-I targeting to the TGN require the binding of Nef to PACS-1, a molecule that controls the TGN localization of the cellular protein furin. This interaction is dependent on Nef’s cluster of acidic amino acids. A chimaeric integral membrane protein containing Nef as its cytoplasmic domain localizes to the TGN after internalization, in an acidic-cluster- and PACS-1-dependent manner. These results support a model in which Nef relocalizes MHC-I by acting as a connector between MHC-I’s cytoplasmic tail and the PACS-1-dependent protein-sorting pathway.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Schwartz, O., Maréchal, V., Le Gall, S., Lemonnier, F. & Heard, J. M. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nature Med. 2, 338–342 (1996).
Collins, K. L., Chen, B. K., Kalams, S. A., Walker, B. D. & Baltimore, D. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature 391, 397–401 (1998).
Greenberg, M. E., Iafrate, A. J. & Skowronski, J. The SH3 domain-binding surface and an acidic motif in HIV-1 Nef regulate trafficking of class I MHC complexes. EMBO J. 17, 2777–2789 (1998).
Wan, L. et al. PACS-1 defines a novel gene family of cytosolic sorting proteins required for trans-Golgi network localization. Cell 94, 205–216 (1998).
Aiken, C., Krause, L., Chen, Y. L. & Trono, D. Mutational analysis of HIV-1 Nef: identification of two mutants that are temperature-sensitive for CD4 downregulation. Virology 217, 293–300 (1996).
Lama, J., Mangasarian, A. & Trono, D. Cell-surface expression of CD4 reduces HIV-1 infectivity by blocking env incorporation in a nef- and vpu-inhibitable manner. Curr. Biol. 9, 622–631 (1999).
Ross, T. M., Oran, A. E. & Cullen, B. R. Inhibition of HIV-1 progeny virion release by cell-surface CD4 is relieved by expression of the viral nef protein. Curr Biol 9, 613–621 (1999).
Aiken, C., Konner, J., Landau, N. R., Lenburg, M. E. & Trono, D. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell 76, 853–864 (1994).
Piguet, V. et al. Nef-induced CD4 degradation: a diacidic-based motif in Nef functions as a lysosomal targeting signal through the binding of β-COP in endosomes. Cell 97, 63–73 (1999).
Rhee, S. S. & Marsh, J. W. Human immunodeficiency virus type 1 Nef-induced down-modulation of CD4 is due to rapid internalization and degradation of surface CD4. J. Virol. 68, 5156–5163 (1994).
Piguet, V. et al. Mechanism of Nef induced CD4 endocytosis: Nef connects CD4 with the µ chain of adaptor complexes. EMBO J. 17, 2472–2481 (1998).
Le Gall, S. et al. Nef interacts with the µ subunit of clathrin adaptor complexes and reveals a cryptic sorting signal in MHC-I molecules. Immunity 8, 483–495 (1998).
Greenberg, M. et al. Co-localization of HIV-1 Nef with the AP-2 adaptor protein complex correlates with Nef-induced CD4 down-regulation. EMBO J. 16, 6964–6976 (1997).
Mangasarian, A. et al. The HIV-1 Nef protein acts as a connector with sorting pathways in the Golgi and at the plasma membrane. Immunity 6, 67–77 (1997).
Craig, H. M., Pandori, M. W. & Guatelli, J. C. Interaction of HIV-1 nef with the cellular dileucine-based sorting pathway is required for CD4 down-regulation and optimal viral infectivity. Proc. Natl Acad. Sci. USA 95, 11229–11234 (1998).
Bresnahan, P. A. et al. A dileucine motif in HIV-1 Nef acts as an internalization signal for CD4 downregulation and binds the AP-1 clathrin adaptor. Curr. Biol. 8, 1235–1238 (1998).
Greenberg, M., DeTulleo, L., Rapoport, I., Skowronski, J. & Kirchhausen, T. A dileucine motif in HIV-1 Nef is essential for sorting into clathrin-coated pits and for downregulation of CD4. Curr. Biol. 8, 1239–1242 (1998).
Demaurex, N., Furuya, W., D’Souza, S., Bonifacino, J. S. & Grinstein, S. Mechanism of acidification of the trans-Golgi network (TGN). In situ measurements of pH using retrieval of TGN38 and furin from the cell surface. J. Biol. Chem. 273, 2044–2051 (1998).
Mukherjee, S., Ghosh, R. N. & Maxfield, F. R. Endocytosis. Physiol. Rev. 77, 759–803 (1997).
Mangasarian, A., Piguet, V., Wang, J. K., Chen, Y. & Trono, D. Nef-induced CD4 and major histocompatibility complex class I (MHC-I) down-regulation are governed by distinct determinants: N-terminal alpha helix and proline repeat of Nef selectively regulate MHC-I trafficking. J. Virol. 73, 1964–1973 (1999).
Baur, A. S. et al. The N-terminus of Nef from HIV-1/SIV associates with a protein complex containing Lck and a serine kinase. Immunity 6, 283–291 (1997).
Saksela, K., Cheng, G. & Baltimore, D. Proline-rich (PxxP) motifs in HIV-1 Nef bind to SH3 domains of a subset of Src kinases and are required for the enhanced growth of Nef+ viruses but not for down-regulation of CD4. EMBO J. 14, 484–491 (1995).
Grzesiek, S., Stahl, S. J., Wingfield, P. T. & Bax, A. The CD4 determinant for downregulation by HIV-1 Nef directly binds to Nef. Mapping of the Nef binding surface by NMR. Biochemistry 35, 10256–10261 (1996).
Ploegh, H. L. Viral strategies of immune evasion. Science 280, 248–253 (1998).
Brodsky, F. M., Lem, L., Solache, A. & Bennett, E. M. Human pathogen subversion of antigen presentation. Immunol Rev. 168, 199–217 (1999).
Acknowledgements
We thank E. Kiyokawa, S. Pfeffer, M. Robinson, G. Nolan and J. Skowronski for providing various reagents and/or for helpful discussions; B. Wehrle-Haller for assistance with imaging; and M. Loche for the artwork. This study was supported by grants from the Gabriella Giorgi-Cavaglieri Foundation and from the Swiss National Science Foundation to D.T., and from the NIH to D.T. and G.T. V.P. was the recipient of an M.D.–Ph.D. scholarship from the Swiss National Science Foundation.
Correspondence and requests for materials should be addressed to D.T. or G.T.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Piguet, V., Wan, L., Borel, C. et al. HIV-1 Nef protein binds to the cellular protein PACS-1 to downregulate class I major histocompatibility complexes. Nat Cell Biol 2, 163–167 (2000). https://doi.org/10.1038/35004038
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/35004038
This article is cited by
-
iPSC-derived models of PACS1 syndrome reveal transcriptional and functional deficits in neuron activity
Nature Communications (2024)
-
WDR37 syndrome: identification of a distinct new cluster of disease-associated variants and functional analyses of mutant proteins
Human Genetics (2021)
-
The multifunctional protein PACS-1 is required for HDAC2- and HDAC3-dependent chromatin maturation and genomic stability
Oncogene (2020)
-
Impaired ability of Nef to counteract SERINC5 is associated with reduced plasma viremia in HIV-infected individuals
Scientific Reports (2020)
-
The autophagy protein ATG9A promotes HIV-1 infectivity
Retrovirology (2019)