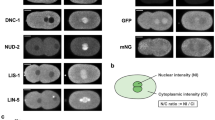
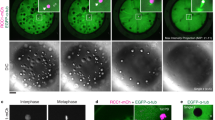
Abstract
Localization of bicoid (bcd) messenger RNA to the anterior pole of the Drosophila oocyte requires the exuperantia ( exu), swallow (swa) and staufen (stau) genes. We show here that Swa protein transiently co-localizes with bcd RNA in mid-oogenesis. Swa also localizes to the anterior pole of the oocyte in the absence of bcd RNA. This localization does not require Exu, but depends on intact microtubules. In mutant ovaries with duplicated polarity of microtubules, Swa and bcd RNA are ectopically localized at the posterior pole, as well as being present at the anterior pole. We identify dynein light chain-1 (Ddlc-1), a component of the minus-end-directed microtubule motor cytoplasmic dynein, as a Swa-binding protein. We propose that Swa acts as an adaptor for the dynein complex and thereby enables dynein to transport bcd RNA along microtubules to their minus ends at the anterior pole of the oocyte.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
St Johnston, D. The intracellular localization of messenger RNAs. Cell 81, 161–170 (1995).
van Eeden, F. & Johnston, D. S. The polarisation of the anterior-posterior and dorsal-ventral axes during Drosophila oogenesis. Curr. Opin. Genet. Dev. 9, 396–404 (1999).
Berleth, T. et al. The role of localization of bicoid RNA in organizing the anterior pattern of the Drosophila embryo. EMBO J. 7, 1749–1756 (1988).
Driever, W. & Nüsslein-Volhard, C. A gradient of Bicoid protein in Drosophila embryos. Cell 54, 83–93 (1988).
Driever, W. & Nüsslein-Volhard, C. The Bicoid protein determines position in the Drosophila embryo in a concentration-dependent manner. Cell 54, 95–104 (1988).
Struhl, G., Struhl, K. & MacDonald, P. The gradient morphogen Bicoid is a concentration-dependent transcriptional activator. Cell 57, 1259 –1273 (1989).
Frigerio, G., Burri, M., Bopp, D., Baumgartner, S. & Noll, M. Structure of the segmentation gene paired and the Drosophila PRD gene set as part of a gene network. Cell 47, 735–746 ( 1986).
Spradling, A. The Development of Drosophila melanogaster (eds Bate, M. & Martinez-Arias, A.) 1–70 (Cold Spring Harb. Lab. Press, Cold Spring Harbor, 1993).
St Johnston, D., Driever, W., Berleth, T., Richstein, S. & Nüsslein-Volhard, C. Multiple steps in the localization of bicoid RNA to the anterior pole of the Drosophila oocyte. Development 107 (Suppl.), 13–19 (1989).
Schüpbach, T. & Wieschaus, E. Maternal-effect mutations altering the anterior-posterior pattern of the Drosophila embryo. Roux’s Arch. Dev. Biol. 195, 302–317 (1986).
Frohnhöfer, H. G. & Nüsslein-Volhard, C. Maternal genes required for the anterior localization of bicoid activity in the embryo of Drosophila. Genes Dev. 1, 880–890 (1987).
Macdonald, P. M., Ka-Shing, S. & Kilpatrick, M. Protein encoded by the exuperantia gene is concentrated at sites of bicoid mRNA accumulation in Drosophila nurse cells but not in oocytes or embryos. Genes Dev. 5, 2455–2466 (1991).
Wilsch-Bräuninger, M., Schwarz, H. & Nüsslein Volhard, C. A sponge-like structure involved in the association and transport of maternal products during Drosophila oogenesis . J. Cell Biol. 139, 817– 829 (1997).
Wang, S. & Hazelrigg, T. Implications for bcd mRNA localization from spatial distribution of exu protein in Drosophila oogenesis. Nature 369, 400– 403 (1994).
Theurkauf, W. E. & Hazelrigg, T. I. In vivo analyses of cytoplasmic transport and cytoskeletal organization during Drosophila oogenesis: characterization of a multi-step anterior localization pathway . Development 125, 3655– 3666 (1998).
Pokrywka, N. J. & Stephenson, E. C. Microtubules mediate the localization of bicoid RNA during Drosophila oogenesis . Development 113, 55–66 (1991).
Pokrywka, N. J. & Stephenson, E. C. Microtubules are a general component of mRNA localization systems in Drosophila oocytes. Dev. Biol. 167, 363– 370 (1995).
Stephenson, E. C., Chao, Y. C. & Fackenthal, J. D. Molecular analysis of the swallow gene of Drosophila-melanogaster. Genes Dev. 2, 1655–1665 (1988).
Chao, Y. C., Donahue, K. M., Pokrywka, N. J. & Stephenson, E. C. Sequence of swallow a gene required for the localization of bicoid message in Drosophila eggs. Dev. Gen. 12 , 333–341 (1991).
Hegde, J. & Stephenson, E. C. Distribution of Swallow protein in egg chambers and embryos of Drosophila melanogaster. Development 119, 457–470 (1993).
Ferrandon, D., Elphick, L., Nüsslein-Volhard, C. & St Johnston, D. Staufen protein associates with the 3’UTR of bicoid mRNA to form particles that move in a microtubule-dependent manner. Cell 79, 1221–1232 (1994).
Payre, F., Crozatier, M. & Vincent, A. Direct control of transcription of the Drosophila morphogen bicoid by the serendipity delta zinc finger protein, as revealed by in vivo analysis of a finger swap. Genes Dev. 8, 2718–2728 (1994).
Theurkauf, W., Alberts, B., Jan, Y. & Jongens, T. A central role of microtubules in the differentiation of Drosophila oocytes. Development 118, 1169–1180 (1993).
Cooley, L. & Theurkauf, W. E. Cytoskeletal functions during Drosophila oogenesis. Science 266, 590 –595 (1994).
Gonzalez-Reyes, A., Elliott, H. & St Johnston, D. Polarization of both major body axes in Drosophila by gurken-torpedo signalling. Nature 375, 654–658 (1995).
King, S. M. et al. Brain cytoplasmic and flagellar outer arm dyneins share a highly conserved Mr 8,000 light chain. J. Biol. Chem. 271, 19358–19366 (1996).
Dick, T., Ray, K., Salz, H. K. & Chia, W. Cytoplasmic dynein (ddlc1) mutations cause morphogenetic defects and apoptotic cell death in Drosophila melanogaster. Mol. Cell. Biol. 16, 1966–1977 (1996).
Phillis, R., Statton, D., Caruccio, P. & Murphey, R. K. Mutations in the 8 kDa dynein light chain gene disrupt sensory axon projections in the Drosophila imaginal CNS. Development 122, 2955–2963 ( 1996).
Vallee, R. B. & Sheetz, M. P. Targeting of motor proteins. Science 271, 1539–1544 ( 1996).
Paschal, B. M. & Vallee, R. B. Retrograde transport by the microtubule-associated protein MAP 1C. Nature 330, 181–183 (1987).
Whittaker, K. L., Ding, D., Fisher, W. W. & Lipshitz, H. D. Different 3’ untranslated regions target alternatively processed hu-li tai shao (hts) transcripts to distinct cytoplasmic locations during Drosophila oogenesis. J. Cell Sci. 112, 3385–3398 (1999).
Ferrandon, D., Koch, I., Westhof, E. & Nüsslein-Volhard, C. RNA-RNA interaction is required for the formation of specific bicoid mRNA 3’ UTR-Staufen ribonucleoprotein particles. EMBO J. 16, 1751–1758 ( 1997).
Karki, S. & Holzbaur, E. L. Cytoplasmic dynein and dynactin in cell division and intracellular transport. Curr. Opin. Cell. Biol. 11, 45–53 ( 1999).
Holleran, E. A., Karki, S. & Holzbaur, E. L. The role of the dynactin complex in intracellular motility. Int. Rev. Cytol. 182, 69– 109 (1998).
McGrail, M. et al. Regulation of cytoplasmic dynein function in vivo by the Drosophila Glued complex. J. Cell. Biol. 131, 411–425 (1995).
Grosshans, J., Schnorrer, F. & Nüsslein-Volhard, C. Oligomerisation of Tube and Pelle leads to nuclear localisation of Dorsal. Mech. Dev. 81, 127–138 (1999).
Lupas, A. Coiled coils: New structures and new functions. Trends Biochem. Sci. 21, 375–382 ( 1996).
Acknowledgements
We thank H. Knaut, S. Luschnig, C. Bökel, J. Müller and J. Großhans for discussions and suggestions; S. King for the anti-Ddlc-1 antibody; J. Shulman for an in situ hybridization protocol; and D. Gilmour, H. Knaut and J. Müller for comments on the manuscript.
Correspondence and requests for materials should be addressed to F.S.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schnorrer, F., Bohmann, K. & Nüsslein-Volhard, C. The molecular motor dynein is involved in targeting Swallow and bicoid RNA to the anterior pole of Drosophila oocytes. Nat Cell Biol 2, 185–190 (2000). https://doi.org/10.1038/35008601
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/35008601
This article is cited by
-
Analysis of ovarian transcriptomes reveals thousands of novel genes in the insect vector Rhodnius prolixus
Scientific Reports (2021)
-
bicoid RNA localization requires the trans-Golgi network
Hereditas (2019)
-
Control of cytoplasmic mRNA localization
Cellular and Molecular Life Sciences (2012)
-
Structure-function-folding relationships and native energy landscape of dynein light chain protein: nuclear magnetic resonance insights
Journal of Biosciences (2009)
-
In silico whole-genome screening for cancer-related single-nucleotide polymorphisms located in human mRNA untranslated regions
BMC Genomics (2007)