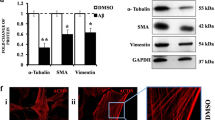
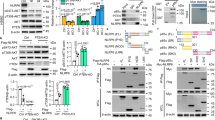
Abstract
The receptor for advanced glycation end products (RAGE), a multi-ligand member of the immunoglobulin superfamily of cell surface molecules1,2, interacts with distinct molecules implicated in homeostasis, development and inflammation, and certain diseases such as diabetes and Alzheimer's disease 3–8. Engagement of RAGE by a ligand triggers activation of key cell signalling pathways, such as p21ras, MAP kinases, NF-κB and cdc42/rac, thereby reprogramming cellular properties9,10,11. RAGE is a central cell surface receptor for amphoterin, a polypeptide linked to outgrowth of cultured cortical neurons derived from developing brain3,12,13,14,15. Indeed, the co-localization of RAGE and amphoterin at the leading edge of advancing neurites indicated their potential contribution to cellular migration, and in pathologies such as tumour invasion. Here we demonstrate that blockade of RAGE–amphoterin decreased growth and metastases of both implanted tumours and tumours developing spontaneously in susceptible mice. Inhibition of the RAGE–amphoterin interaction suppressed activation of p44/p42, p38 and SAP/JNK MAP kinases; molecular effector mechanisms importantly linked to tumour proliferation, invasion and expression of matrix metalloproteinases16,17,18,19,20,21,22,23.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Schmidt, A. M. et al. Isolation and characterization of binding proteins for advanced glycosylation endproducts from lung tissue which are present on the endothelial cell surface. J. Biol. Chem. 267, 14987– 14997 (1992).
Neeper, M. et al. Cloning and expression of RAGE: a cell surface receptor for advanced glycosylation end products of proteins. J. Biol. Chem. 267, 14998–15004 (1992).
Hori, O. et al. The receptor for advanced glycation endproducts (RAGE) is a cellular binding site for amphoterin: mediation of neurite outgrowth and coexpression of RAGE and amphoterin in the developing nervous system. J. Biol. Chem. 270, 25752–25761 ( 1995).
Wautier, J.-L et al. Receptor-mediated endothelial cell dysfunction in diabetic vasculopathy: soluble receptor for advanced glycation endproducts blocks hyperpermeability. J. Clin. Invest. 97, 238– 243 (1996).
Park, L. et al. Suppression of accelerated diabetic atherosclerosis by soluble receptor for AGE (sRAGE). Nature Med. 4,1025–1031 (1998).
Yan, S. D. et al. RAGE and amyloid beta peptide neurotoxicity in Alzheimer's disease. Nature 382, 685–691 (1996).
Yan, S. D. et al. Amyloid-beta peptide-RAGE interaction elicits neuronal expression of M-CSF: a proinflammatory pathway in Alzheimer's disease. Proc. Natl Acad. Sci. USA 94, 5296–5301 (1997).
Hofmann, M. A. et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell 97, 889–901 (1999).
Yan, S.-D. et al. Enhanced cellular oxidant stress by the interaction of advanced glycation endproducts with their receptors/binding proteins. J. Biol. Chem. 269, 9889–9897 (1994).
Lander, H. L., Tauras, J. M., Ogiste, J. S., Moss, R. A. & Schmidt, A. M. Activation of the receptor for advanced glycation endproducts triggers a MAP kinase pathway regulated by oxidant stress. J. Biol. Chem. 272, 17810 –17814 (1997).
Huttunen, H. J., Fages, C. & Rauvala, H. Receptor for advanced glycation endproducts (RAGE)-mediated neurite outgrowth and activation of NF-κB require the cytoplasmic domain of the receptor but different downstream signaling pathways. J. Biol. Chem. 274, 19919–19924 (1999).
Rauvala, H. & Pihlaskari, R. Isolation and some characteristics of an adhesive factor of brain that enhances neurite outgrowth in central neurons. J. Biol. Chem. 262,16625–16635 (1987).
Rauvala, H. et al. The adhesive and neurite-promoting molecule p30: analysis of the amino-terminal sequence and production of antipeptide antibodies that detect p30 at the surface of neuroblastoma cells and of brain neurons. J. Cell. Biol. 107, 2293–22305 (1988).
Parkkinen, J. & Rauvala, H. Interactions of plasminogen and tissue plasminogen activator (t-PA) with amphoterin. J. Biol. Chem. 266, 16730–16735 ( 1991).
Parkkinen, J. et al. Amphoterin, the 30-kDa protein in a family of HMG1-type polypeptides. Enhanced expression in transformed cells, leading edge localization, and interactions with plasminogen activation. J. Biol. Chem. 268,19726–19738 (1993).
Brunet, A. et al. Nuclear translocation of p42/p44 mitogen-activated protein kinase is required for growth factor-induced gene expression and cell cycle entry. EMBO J. 18, 664– 674 (1999).
Tsang, D. K. & Crowe, D. L. The mitogen activated protein kinase pathway is required for proliferation but not invasion of human squamous cell carcinoma lines. Int. J. Oncol. 15, 519– 523 (1999).
Talarmin, H. et al. The mitogen-activated protein kinase/extracellular signal-regulated kinase cascade activation is a key signalling pathway involved in the regulation of G(1) phase progression in proliferating hepatocytes. Mol. Cell. Biol. 19, 6003–6011 ( 1999).
Klemke, R. L. et al. Regulation of cell motility by mitogen-activated protein kinase. J. Cell Biol. 137, 481– 492 (1997).
Montesano, R., Soriano, J. V., Hossseini, G., Pepper, M. S. & Schramek, H. Constitutively active mitogen-activated protein kinase MEK1 disrupts morphogenesis and induces an invasive phenotype in Madin-Darby canine kidney epithelial cells. Cell Growth Differ. 10, 317–332 ( 1999).
Reddy, K. B., Krueger, J. S., Kondapaka, S. B. & Diglio, C. A. Mitogen-activated protein kinase (MAPK) regulates the expression of progelatinase B (MMP-9) in breast epithelial cells. Int. J. Cancer 82, 268–273 (1999).
Aguirre-Ghiso, J. A. et al. RalA requirement for v-Src- and v-Ras-induced tumorigenicity and overproduction of urokinase-type plasminogen activator: involvement of metalloproteases. Oncogene 18, 4718– 4725 (1999).
Esparza, J. et al. Fibronectin upregulates gelatinase B (MMP-9) and induces coordinated expression of gelatinase A (MMP-2) and its activator MT1-MMP (MMP-14) by human T lymphocyte cell lines. A process repressed through Ras/MAP kinase signaling pathways. Blood 94, 2754– 2766 (1999).
Brett, J. et al. Tissue distribution of the receptor for advanced glycation endproducts (RAGE): expression in smooth muscle, cardiac myocytes, and neural tissue in addition to the vasculature. Am. J. Pathol. 143,1699–1712 (1993).
O'Reilly, M. S. et al. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell 79, 315–328 (1994).
Leder, A., Kuo, A., Cardiff, R. D., Sinn, E. & Leder, P. V-Ha-ras transgene abrogates the initiation step in mouse skin tumorigenesis: effects of phorbol esters and retinoic acid. Proc. Natl Acad. Sci. USA 87, 9178–9182 ( 1990).
Yu, W., Kim, J. & Ossowski, L. Reduction in surface urokinase receptor forces malignant cells into a protracted state of dormancy. J. Cell Biol. 137, 767–777 (1997).
Valente, P. et al. TIMP-2 over-expression reduces invasion and angiogenesis and protects B16F10 melanoma cells from apoptosis. Int. J. Cancer 75, 246–253 (1998).
Albini, A. et al. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res. 47, 3239–3245 (1987).
Ramamurthy, N. S. & Golub, L. M. Diabetes increases collagenase activity in extracts of rat gingiva and skin. J. Periodont. Res. 18, 23–30 ( 1983).
Acknowledgements
We thank P. D'Amore for advice and B. Tycko for assistance in these studies. This work was supported by the Surgical Research Fund of the College of Physicians & Surgeons, Columbia University, and by grants from the United States Public Health Service, Juvenile Diabetes Foundation International, and the American Heart Association, New York affiliate. G.D.T. is a recipient of a Faculty Development Award from the Robert Wood Johnson Foundation. A.M.S. is a recipient of a Burroughs Wellcome Fund Clinical Scientist Award in Translational Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Taguchi, A., Blood, D., del Toro, G. et al. Blockade of RAGE–amphoterin signalling suppresses tumour growth and metastases. Nature 405, 354–360 (2000). https://doi.org/10.1038/35012626
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35012626
This article is cited by
-
Associations of circulating advanced glycation end products and their soluble receptors with cancer risk: A systematic review and meta-analysis of observational studies
Glycoconjugate Journal (2024)
-
RAGE inhibitor TTP488 (Azeliragon) suppresses metastasis in triple-negative breast cancer
npj Breast Cancer (2023)
-
The multifunctional protein HMGB1: 50 years of discovery
Nature Reviews Immunology (2023)
-
Nε-(1-Carboxymethyl)-L-lysine, an advanced glycation end product, exerts malignancy on chondrosarcoma via the activation of cancer stemness
Archives of Toxicology (2023)
-
RAGE signaling during tobacco smoke-induced lung inflammation and potential therapeutic utility of SAGEs
BMC Pulmonary Medicine (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.