Abstract
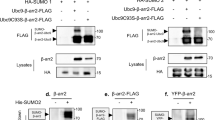
MDM2 can bind to p53 and promote its ubiquitination and subsequent degradation by the proteasome. Current models propose that nuclear export of p53 is required for MDM2-mediated degradation, although the function of MDM2 in p53 nuclear export has not been clarified. Here we show that MDM2 can promote the nuclear export of p53 in transiently transfected cells. This activity requires the nuclear-export signal (NES) of p53, but not the NES of MDM2. A mutation within the MDM2 RING-finger domain that inhibits p53 ubiquitination also inhibits the ability of MDM2 to promote p53 nuclear export. Finally, inhibition of nuclear export stabilizes wild-type p53 and leads to accumulation of ubiquitinated p53 in the nucleus. Our results indicate that MDM2-mediated ubiquitination, or other activities associated with the RING-finger domain, can stimulate the export of p53 to the cytoplasm through the activity of the p53 NES.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Haupt, Y., Maya, R., Kazaz, A. & Oren, M. Mdm2 promotes the rapid degradation of p53. Nature 387, 296–299 (1997).
Kubbutat, M. H., Jones, S. N. & Vousden, K. H. Regulation of p53 stability by MDM2. Nature 387, 299–303 (1997).
Kudo, N. et al. Leptomycin B inhibition of signal-mediated nuclear export by direct binding to CRM1. Exp. Cell Res. 242, 540–547 (1998).
Freedman, D. A. & Levine, A. J. Nuclear export is required for degradation of endogenous p53 by MDM2 and human papillomavirus E6. Mol. Cell Biol. 18, 7288–7293 (1998).
Stommel, J. M. et al. A leucine-rich nuclear export signal in the p53 tetramerization domain: regulation of subcellular localization and p53 activity by NES masking. EMBO J. 18, 1660–1672 (1999).
Roth, J., Dobbelstein, M., Freedman, D. A., Shenk, T. & Levine, A. J. Nucleo-cytoplasmic shuttling of the hdm2 oncoprotein regulates the levels of the p53 protein via a pathway used by the human immunodeficiency virus rev protein. EMBO J. 17, 554–564 (1998).
Chen, J., Marechal, V. & Levine, A. J. Mapping of the p53 and mdm-2 interaction domains. Mol. Cell Biol. 13, 4107–4114 (1993).
Unger, T., Mietz, J. A., Scheffner, M., Yee, C. L. & Howley, P. M. Functional domains of wild-type and mutant p53 proteins involved in transcriptional regulation, transdominant inhibition, and transformation suppression. Mol. Cell Biol. 13, 5186–5194 (1993).
Ellenbaas, B., Dobblestein, M., Roth, J., Shenk, T. & Levine, A. J. The MDM2 oncoprotein binds specifically to RNA through its RING finger domain. Mol. Med. 2, 439–451 (1996).
Honda, R., Tanaka, H. & Yasuda H. Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53. FEBS Lett. 420, 25–27 (1997).
Taminura, S. et al. MDM2 interacts with MDMX through their RING finger domains. FEBS Lett. 447, 5–9 (1999).
Kubbutat, M. H., Ludwig, R. L., Levine, A. J. & Vousden, K. H. Analysis of the degradation function of Mdm2. Cell Growth Diff. 10, 87–92 (1999).
Maki, C. G., Huibregtse, J. M. & Howley, P. M. In vivo ubiquitination and proteasome-mediated degradation of p53. Cancer Res. 56, 2649–2654 (1996).
Olivier, M., Bautista, S., Valles, H. & Theillet, C. Relaxed cell-cycle arrests and propagation of unrepaired chromosomal damage in cancer cell lines with wild-type p53. Mol. Carcinogenesis 23, 1–12 (1998).
Kamura, T. et al. Rbx1, a component of the VHL tumor suppressor complex and SCF ubiquitin ligase. Science 284, 657–661 (1999).
Skowyra, D. et al. Reconstitution of G1 cyclin ubiquitination with complexes containing SCF grr and Rbx1. Science 284, 662–665 (1999).
Gostissa, M. et al. Activation of p53 by conjugation to the ubiquitin-like protein SUMO-1. EMBO J. 18, 6462–6471 (1999).
Rodriguez, M. S. et al. SUMO-1 modification activates the transcriptional response of p53. EMBO J. 18, 6455–6461 (1999).
Marechal, V., Elenbaas, B., Piette, J., Nicolas, J. C. & Levine, A. J. The ribosomal L5 protein is associated with mdm-2 and mdm-2–p53 complexes. Mol. Cell Biol. 14, 7414–7420 1994.
Kamijo, T. et al. Functional and physical interactions of the ARF tumor suppressor with p53 and MDM2. Proc. Natl Acad. Sci. USA 95, 8292–8297 (1998).
Honda, R. & Yasuda, H. Association of p19 ARF with MDM2 inhibits ubiquitin ligase activity of MDM2 for tumor suppressor p53. EMBO J. 18, 22–27 (1999).
Tao, W. & Levine, A. J. p19 (ARF) stabilizes p53 by blocking nucleo-cytoplasmic shuttling of MDM2. Proc. Natl Acad. Sci. USA 96, 6937–6941 (1999).
Zhang, Y. & Xiong, Y. Mutations in human ARF exon two disrupt its nucleolar localization and impair its ability to block nuclear export of MDM2 and p53. Mol. Cell 3, 579–591 (1999).
Weber, J. D., Taylor, J. D., Roussel, M. F., Sherr, C. J. & Bar-Sagi, D. Nucleolar Arf sequesters Mdm2 and activates p53. Nature Cell Biol. 1, 20–26 (1999).
Levine, A. J. p53, the cellular gatekeeper for growth and division. Cell 88, 323–331 (1997).
Sakaguchi, K. et al. Phosphorylation of serine 392 stabilizes the tetramer formation of tumor suppressor protein p53. Biochemistry 36, 10117–10124 (1997).
Unger, T., Mietz, J. A., Scheffner, M., Yee, C. L. & Howley, P. M. Functional domains of wild-type and mutant p53 proteins involved in transcriptional regulation, transdominant inhibition, and transformation suppression. Mol. Cell Biol. 13, 5186–5194 (1993).
Acknowledgements
We thank P. Howley and P. Hinds for advice and critical reading of the manuscript. This work was supported by Public Health Service grant 1R01CA80918 and by a breast cancer research grant from the Massachusetts Department of Public Health (both to C.G.M.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Geyer, R., Yu, Z. & Maki, C. The MDM2 RING-finger domain is required to promote p53 nuclear export. Nat Cell Biol 2, 569–573 (2000). https://doi.org/10.1038/35023507
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/35023507
This article is cited by
-
DNA damage response revisited: the p53 family and its regulators provide endless cancer therapy opportunities
Experimental & Molecular Medicine (2022)
-
MYC: a multipurpose oncogene with prognostic and therapeutic implications in blood malignancies
Journal of Hematology & Oncology (2021)
-
Adipose MDM2 regulates systemic insulin sensitivity
Scientific Reports (2021)
-
ZCCHC10 suppresses lung cancer progression and cisplatin resistance by attenuating MDM2-mediated p53 ubiquitination and degradation
Cell Death & Disease (2019)
-
Structural analysis of MDM2 RING separates degradation from regulation of p53 transcription activity
Nature Structural & Molecular Biology (2017)