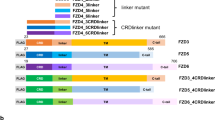
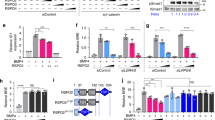
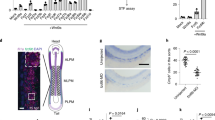
Abstract
The Wnt family of secreted signalling molecules are essential in embryo development and tumour formation1. The Frizzled (Fz) family of serpentine receptors function as Wnt receptors2,3,4,5,6,7,8,9,10, but how Fz proteins transduce signalling is not understood. In Drosophila , arrow phenocopies the wingless (DWnt-1) phenotype11, and encodes a transmembrane protein11 that is homologous to two members of the mammalian low-density lipoprotein receptor (LDLR)-related protein (LRP) family, LRP5 and LRP6 (refs 12,13,14, 15). Here we report that LRP6 functions as a co-receptor for Wnt signal transduction. In Xenopus embryos, LRP6 activated Wnt–Fz signalling, and induced Wnt responsive genes, dorsal axis duplication and neural crest formation. An LRP6 mutant lacking the carboxyl intracellular domain blocked signalling by Wnt or Wnt–Fz, but not by Dishevelled or β-catenin, and inhibited neural crest development. The extracellular domain of LRP6 bound Wnt-1 and associated with Fz in a Wnt-dependent manner. Our results indicate that LRP6 may be a component of the Wnt receptor complex.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Wodarz, A. & Nusse, R. Mechanisms of Wnt signalling in development. Annu. Rev. Cell Dev. Biol. 14, 59– 88 (1998).
Bhanot, P. et al. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature 382, 225– 230 (1996).
Yang-Snyder, J., Miller, J. R., Brown, J. D., Lai, C. J. & Moon, R. T. A frizzled homolog functions in a vertebrate Wnt signalling pathway. Curr. Biol. 6, 1302–1306 (1996).
He, X. et al. A member of the Frizzled protein family mediating axis induction by Wnt-5A. Science 275, 1652– 1654 (1997).
Bhat, K. M. frizzled and frizzled 2 play a partially redundant role in wingless signalling and have similar requirements to wingless in neurogenesis. Cell 95, 1027–1036 ( 1998).
Kennerdell, J. R. & Carthew, R. W. Use of dsRNA-mediated genetic interference to demonstrate that frizzled and frizzled 2 act in the wingless pathway. Cell 95, 1017– 1026 (1998).
Muller, H., Samanta, R. & Wieschaus, E. Wingless signalling in the Drosophila embryo: zygotic requirements and the role of the frizzled genes. Development 126, 577–586 ( 1999).
Hsieh, J. C., Rattner, A., Smallwood, P. M. & Nathans, J. Biochemical characterization of Wnt-frizzled interactions using a soluble, biologically active vertebrate Wnt protein. Proc. Natl Acad. Sci. USA 96, 3546–3551 ( 1999).
Bhanot, P. et al. Frizzled and Dfrizzled-2 function as redundant receptors for Wingless during Drosophila embryonic development. Development 126, 4175–4186 ( 1999).
Chen, C. M. & Struhl, G. Wingless transduction by the Frizzled and Frizzled2 proteins of Drosophila. Development 126, 5441–5452 (1999).
Wehrli, M. et al. arrow encodes an LDL-receptor-related protein essential for Wingless signalling. Nature 407, 527 –530 (2000).
Brown, S. D. et al. Isolation and characterization of LRP6, a novel member of the low density lipoprotein receptor gene family. Biochem. Biophys. Res. Commun. 248, 879–888 (1998).
Hey, P. J. et al. Cloning of a novel member of the low-density lipoprotein receptor family. Gene 216, 103–111 (1998).
Kim, D. H. et al. A new low density lipoprotein receptor related protein, LRP 5, is expressed in hepatocytes and adrenal cortex, and recognizes apolipoprotein E. J. Biochem. (Tokyo) 124, 1072– 1076 (1998).
Dong, Y. et al. Molecular cloning and characterization of LR3, a novel LDL receptor family protein with mitogenic activity. Biochem. Biophys. Res. Commun. 251, 784–790 ( 1998).
Pinson, K. I., Brennan, J., Monkley, S., Avery, B & Skarnes, W. C. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature 407, 535– 538 (2000).
Harland, R. M. & Gerhart, J. Formation and function of Spemann's organizer. Annu. Rev. Cell Dev. Biol. 13, 611–667 (1997).
Ikeya, M., Lee, S. M. K., Johnson, J. E., McMahon, A. P. & Takada, S. Wnt signalling required for expansion of neural crest and CNS progenitors. Nature 389, 966–970 (1997).
Saint-Jeannet, J.-P., He, X., Varmus, H. E. & Dawid, I. B. Regulation of dorsal fate in the neuraxis by Wnt-1 and Wnt-3a. Proc. Natl Acad. Sci. USA 94, 13713–13718 (1997).
Chang, C. & Hemmati-Brivanlou, A. Neural crest induction by Xwnt7B in Xenopus. Dev. Biol. 194, 129–134 (1998).
LaBonne, C. & Bronner-Fraser, M. Neural crest induction in Xenopus: evidence for a two signal model. Development 125, 2403–2414 (1998).
Dorsky, R. I., Moon, R. T. & Raible, D. W. Control of neural crest cell fate by the Wnt signalling pathway. Nature 396, 370– 373 (1998).
Sumanas, S., Strege, P., Heasman, J., & Ekker, S. C. The putative Wnt receptor Xenopus frizzled-7 functions upstream of β-catenin in vertebrate dorsoventral mesoderm patterning. Development 127, 1981–1990 (2000).
Zeng, L. et al. The mouse fused locus encodes Axin, an inhibitor of the Wnt signalling pathway that regulates embryonic axis formation. Cell 90, 181–192 (1997).
Molenaar, M. et al. XTcf-3 transcription factor mediates β-catenin-induced axis formation in Xenopus embryos. Cell 86, 391–399 (1996).
Finch, P. W. et al. Purification and molecular cloning of a secreted, Frizzled-related antagonist of Wnt action. Proc. Natl Acad. Sci. USA 94, 6770–6775 (1997).
Perrimon, N. & Bernfield, M. Specificities of heparan sulphate proteoglycans in developmental processes. Nature 404 , 725–728 (2000).
Trommsdorff, M. et al. Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell 97, 689–701 (1999).
Kato, Y., Shi, Y. & He, X. Neuralization of the Xenopus embryo by inhibition of p300/CBP function. J. Neuroscience 19, 9346– 9373 (1999).
Shimizu, H. et al. Transformation by Wnt family proteins correlates with regulation of β-catenin. Cell Growth Differ. 8, 1349–1358 (1997).
Acknowledgements
We thank M. Semenova for technical assistance; J. Heitz, J. Kitajewski, J. Nathans, S. Sokol, D. Sussman and A. Parlow (NHPP) for reagents; S. DiNardo and B. Skarnes for communication; and R. Habas, Z. He and Q. Ma for comments. X.H. acknowledges supports from Johnson and Johnson, the US Army, Susan G. Komen Foundation and the NIH. J.-P.S.-J. acknowledges supports from Johnson and Johnson and Whitehall Foundation. X.H. is a Pew Scholar and Klingenstein Fellow.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tamai, K., Semenov, M., Kato, Y. et al. LDL-receptor-related proteins in Wnt signal transduction. Nature 407, 530–535 (2000). https://doi.org/10.1038/35035117
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35035117
This article is cited by
-
The scaffold protein AXIN1: gene ontology, signal network, and physiological function
Cell Communication and Signaling (2024)
-
Design, synthesis, and in silico studies of novel di-(2-aryl hydrozonopropanal) arene derivatives as potent anticancer for targeting A2AR and LRP6 in HCT116 cell
Medicinal Chemistry Research (2024)
-
IGFBP2 drives epithelial-mesenchymal transition in hepatocellular carcinoma via activating the Wnt/β-catenin pathway
Infectious Agents and Cancer (2023)
-
The WNT/β-catenin system in chronic kidney disease-mineral bone disorder syndrome
International Urology and Nephrology (2023)
-
Cellular signaling crosstalk between Wnt signaling and gap junctions inbenzo[a]pyrene toxicity
Cell Biology and Toxicology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.