Abstract
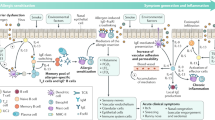
Recent studies have shown that initial sensitization to airborne environmental allergens occurs typically in early childhood, but subsequent progression to persistent atopic asthma, which may not manifest for several years, is restricted to only a subset of atopics. The key to establishing the link between atopy and asthma lies in the development of persistent inflammation in the airway wall, resulting in structural and functional changes in local tissues which are responsible for the symptoms of the disease. This review summarizes recent findings on the nature of the cellular and molecular mechanisms underlying this process, and addresses the issue of why the intensity and duration of these tissue-damaging responses in the airway wall apparently exceeds the critical threshold required for development of persistent asthma in only a minority of allergy sufferers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Notes
*Terms in italic are defined in the glossary on p. 39.
References
Barnes, P. J., Chung, K. F. & Page, C. P. Inflammatory mediators of asthma: an update. Pharmacol. Rev. 50, 515–596 (1998).
O'Byrne, P. M. Leukotrienes in the pathogenesis of asthma. Chest 111 , 27S–34S (1997).
Galli, S. J. & Costa, J. J. Mast-cell-leukocyte cytokine cascades in allergic inflammation. Allergy 50, 851 –862 (1995).
Coffman, R. L. et al. The role of helper T cell products in mouse B cell differentiation and isotype regulation. Immunol. Rev. 102, 5–28 (1988).
Hogan, S. P., Koskinen, A., Matthaei, K. I., Young, I. G. & Foster, P. S. IL-5 producing CD4+ T-cells play a pivotal role in the induction of eosinophilia and allergic airways disease in mice. Am. J. Respir. Crit. Care Med. 157, 210–218 (1998).
Kay, A. B. in Allergy and Allergic Diseases (ed. Kay, A. B. ) 23– 35 (Blackwell Science, Oxford, 1997).
Dugas, B. et al. Interleukin-9 potentiates the interleukin-4-induced immunoglobulin (IgG, IgM and IgE) production by normal human B lymphocytes. Eur. J. Immunol. 23, 1687–1692 (1993).
Petit-Frere, C., Dugas, B., Braquet, P. & Mencia-Huerta, J. M. Interleukin-9 potentiates the interleukin-4-induced IgE and IgG1 release from murine B lymphocytes. Immunology 79, 146–151 (1993).
Kung, T. T., Stelts, D. & Zurcher, J. A. Mast cells modulate allergic pulmonary eosinophilia in mice. Am. J. Respir. Cell Mol. Biol. 12, 404–409 (1995).
Wershil, B. K., Wang, Z. -S., Gordon, J. R. & Galli, S. J. Recruitment of neutrophils during IgE-dependent cutaneous late phase reactions in the mouse is mast cell-dependent. J. Clin. Invest. 87, 446–453 (1991).
Wierenga, E. A. et al. Evidence for compartmentalization of functional subsets of CD2+ T lymphocytes in atopic patients. J. Immunol. 144, 4651–4656 ( 1990).
Romagnani, S. Human TH1 and TH2 subsets: doubt no more. Immunol. Today 12, 256–257 (1991).
Essayan, D. M., Han, W. -F., Xiao, H. -Q., Kleine-Tebbe, J. & Huang, S. -K. Clonal diversity of IL-4 and IL-13 expression in human allergen-specific T lymphocytes. J. Allergy Clin. Immunol. 98, 1035–1044 (1996).
Byron, K. A., O'Brien, R. M., Varigos, G. A. & Wootton, A. M. Dermatophagoides pteronyssinus II-induced interleukin-4 and interferon-γ expression by freshly isolated lymphocytes of atopic individuals. Clin. Exp. Allergy 24, 878–883 (1994).
Prescott, S. L. et al. Transplacental priming of the human immune system to environmental allergens: universal skewing of initial T-cell responses towards the Th-2 cytokine profile. J. Immunol. 160, 4730– 4737 (1998).
Wegmann, T. G., Lin, H., Guilbert, L. & Mosmann, T. R. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a Th2 phenomenon? Immunol. Today 14, 353 –356 (1993).
Holt, P. G. & Macaubas, C. Development of long term tolerance versus sensitisation to environmental allergens during the perinatal period. Curr. Opin. Immunol. 9, 782– 787 (1997).
Holt, P. G. et al. T-cell “priming” against environmental allergens in human neonates: sequential deletion of food antigen specificities during infancy with concomitant expansion of responses to ubiquitous inhalant allergens. Pediatr. Allergy Immunol. 6, 85– 90 (1995).
Yabuhara, A. et al. Th-2-polarised immunological memory to inhalant allergens in atopics is established during infancy and early childhood. Clin. Exp. Allergy 27, 1261–1269 (1997).
Prescott, S. L. et al. Development of allergen-specific T-cell memory in atopic and normal children. Lancet 353, 196– 200 (1999).
Holt, P. G. Environmental factors and primary T-cell sensitisation to inhalant allergens in infancy: reappraisal of the role of infections and air pollution. Pediatr. Allergy Immunol. 6, 1–10 (1995).
Holt, P. G. et al. Genetic ‘risk’ for atopy is associated with delayed postnatal maturation of T-cell competence. Clin. Exp. Allergy 22, 1093–1099 (1992).
Holt, P. G., Sly, P. D. & Björkstén, B. Atopic versus infectious diseases in childhood: a question of balance? Pediatr. Allergy Immunol. 8, 53–58 (1997).
Strachan, D. P. Hay fever, hygiene, and household size. Br. Med. J. 299, 1259–1260 (1989).
Martinez, F. D. Role of viral infections in the inception of asthma and allergies during childhood: could they be protective? Thorax 49, 1189 –1191 (1994).
Baldini, M. et al. A polymorphism in the 5′-flanking region of the CD14 gene is associated with circulating soluble CD14 levels with total serum IgE. Am. J. Respir. Cell Mol. Biol. 20, 976– 983 (1999).
Landau, L. I. in Pediatric Respiratory Medicine (ed. Taussig, L. M. et al.) 935–938 (Mosby, St Louis, 1999).
Johnston, S. L. Viruses and asthma. Allergy 53, 922– 932 (1998).
Macklem, P. T. Bronchial hyporesponsiveness. Chest 87, 158S–159S (1985).
Kraft, M., Djukanovic, R., Wilson, S., Holgate, S. T. & Martin, R. J. Alveolar tissue inflammation in asthma. Am. J. Respir. Crit. Care Med. 154, 1505–1510 (1996).
Ohuri, T. et al. Partitioning of pulmonary responses to inhaled methacholine in subjects with asymptomatic asthma. Am. Rev. Respir. Dis. 146, 1501–1505 (1992).
Sly, P. D. & Lanteri, C. J. Partitioning of pulmonary responses to inhaled methacholine in puppies. J. Appl. Physiol. 71, 886–891 (1991).
Hantos, Z., Petak, F., Adamicza, A., Daroczy, B. & Fredberg, J. J. Differential responses of global airway, terminal airway and tissue impedances to histamine. J. Appl. Physiol. 79, 1440–1448 (1995).
Sly, P. D., Hayden, M. J., Petak, F. & Hantos, Z. Measurement of low-frequency respiratory impedance in infants. Am. J. Respir. Crit. Care Med. 154, 161–166 (1996).
Armour, C. et al. Mediators on human airway smooth muscle. Clin. Exp. Pharmacol. Physiol. 24, 269–272 (1997).
Brewster, C. E. P. et al. Myofibroblasts and subepithelial fibrosis in bronchial asthma. Am. J. Respir. Crit. Care Med. 3, 507– 511 (1990).
Gizycki, M. J., Adelröth, E., Rogers, A. V., O'Byrne, P. M. & Jeffrey, P. K. Myofibroblast involvement in the allergen-induced late response in mild atopic asthma. Am. J. Respir. Crit. Care Med. 16, 664–673 (1997).
Zhang, S., Smartt, H., Holgate, S. T. & Roche, W. R. Growth factors secreted by bronchial epithelial cells control myofibroblast proliferation: an in vitro co-culture model of airway remodelling in asthma. Lab. Invest. 79, 395–405 (1999).
Foster, P. S. H. S., Ramsay, A. J., Matthaei, K. I. & Young, I. G. Interleukin 5 deficiency abolishes eosinophilia, airways hyperreactivity, and lung damage in a mouse asthma model. J. Exp. Med. 183, 195–201 (1996).
Renz, H. et al. Specific Vb T cell subsets mediate the immediate hypersensitivity response to ragweed allergen. J. Immunol. 151, 1907–1917 (1993).
Mishima, H., Hojo, M., Watanade, A., Hamid, Q. & Martin, J. G. CD4+ T cells can induce airway hyperresponsiveness to allergen challenge in the brown Norway rat. Am. J. Respir. Crit. Care Med. 158, 1863–1870 (1998).
Lee, J. J. et al. Interleukin-5 expression in the lung epithelium of transgenic mice leads to pulmonary changes pathognomonic of asthma. J. Exp. Med. 185, 2143–2156 ( 1997).
Tang, W. et al. Targeted expression of IL-11 in the murine airway causes lymphocytic inflammation, bronchial remodelling, and airways obstruction. J. Clin. Invest. 98, 2845–2853 (1996).
DiCosmo, B. F. et al. Airway epithelial cell expression of interleukin-6 in transgenic mice. Uncoupling of airway inflammation and bronchial hyperreactivity. J. Clin. Invest. 94, 2028–2035 (1994).
Knight, D. H., Lydell, C. P., Zhou, D., Schellenberg, R. R. & Bai T. R. Distribution of leukaemia inhibitory factor (LIF) and its receptor in lung. Effect of IL-1β and IL-6 on gene expression and LIF release. Am. J. Respir. Cell Mol. Biol. 20, 834– 841 (1999).
Grunig, G. W. M. et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science 282, 2261– 2263 (1998).
Wills-Karp, M. et al. Interleukin-13: central mediator of allergic asthma. Science 282, 2258–2261 ( 1998).
Woolcock, A. J., Peat, J. K. & Trevillion, L. M. Is the increase in asthma prevalence linked to increase in allergen load? Allergy 50, 935– 940 (1995).
Johnston, S. L. et al. The relationship between upper respiratory infections and hospital admissions for asthma: a time-trend analysis. Am. J. Respir. Crit. Care Med. 154, 654–660 (1996).
Peat, J. K. et al. House dust mite allergens: a major risk factor for childhood asthma in Australia. Am. J. Respir. Crit. Care Med. 153, 141–146 (1996).
Michel, O. et al. Dose-response relationship to inhaled endotoxin in normal subjects. Am. J. Respir. Crit. Care Med. 156, 1157–1164 (1997).
Von Mutius, E. Progression of allergy and asthma through childhood to adolescence. Thorax S1, S3–S6 ( 1996).
Bingisser, R. M., Tilbrook, P. A., Holt, P. G. & Kees, U. R. Macrophage-derived nitric oxide regulates T-cell activation via reversible disruption of the Jak3/Stat5 signalling pathway. J. Immunol. 160, 5729–5734 (1998).
Liew, F. Y. Regulation of lymphocyte functions by nitric oxide. Curr. Opin. Immunol. 7, 396–399 ( 1995).
Barnes, P. J. Reactive oxygen species and airway inflammation. Free Radical Biol. Med. 9, 235–253 ( 1990).
Holt, P. G. et al. Downregulation of the antigen presenting cell function(s) of pulmonary dendritic cells in vivo by resident alveolar macrophages. J. Exp. Med. 177, 397–407 (1993).
Stumbles, P. A. et al. Resting respiratory tract Dendritic Cells preferentially stimulate Th2 responses and require obligatory cytokine signals for induction of Th1 immunity. J. Exp. Med. 188, 2019– 2031 (1998).
Poston, R. N. et al. Immunohistochemical characterisation of the cellular infiltration in asthmatic bronchi. Am. Rev. Respir. Dis. 145, 918–921 (1992).
Moller, G. M. et al. Increased numbers of dendritic cells in the bronchial mucosa of atopic asthmatic patients: downregulation by inhaled corticosteroids. Clin. Exp. Allergy 26, 517–524 (1996).
Godthelp, T. et al. Antigen presenting cells in the nasal mucosa of patients with allergic rhinitis during allergen provocation. Clin. Exp. Allergy 26, 677–688 ( 1996).
Tunon-de-lara, J. M. et al. Dendritic cells in normal and asthmatic airways: expression of the α subunit of the high affinity immunoglobulin E receptor (FcεRI-α). Clin. Exp. Allergy 26, 648–655 (1996).
Lambrecht, B. N., Salomon, B., Klatzmann, D. & Pauwels, R. A. Dendritic cells are required for the development of chronic eosinophilic airway inflammation in response to inhaled antigen in sensitized mice. J. Immunol. 160, 4090–4097 (1998).
Strickland, D. H., Kees, U. R. & Holt, P. G. Regulation of T-cell activation in the lung: alveolar macrophages induce reversible T-cell anergy in vivo associated with inhibition of IL-2R signal transduction. Immunology 87, 250–258 (1996).
Poulter, L. W., Janossy, G., Power, C., Sreenan, S. & Burke, C. Immunological/physiological relationships in asthma: potential regulation by lung macrophages. Immunol. Today 15, 258–261 (1994).
Borish, L. & Rosenwasser, L. TH1//TH2 lymphocytes: doubt some more. J. Allergy Clin. Immunol. 99, 161–164 (1997).
Borish, L. et al. Interleukin-10 regulation in normal subjects and patients with asthma. J. Allergy Clin. Immunol. 97, 1288–1296 (1996).
Macaubas, C. et al. Regulation of Th-cell responses to inhalant allergen during early childhood. Clin. Exp. Allergy 29, 1223–1231 (1999).
Rott, L. S. et al. Expression of mucosal homing receptor α4β7 by circulating CD4+cells with memory for intestinal rotavirus. J. Clin. Invest. 100, 1204–1208 (1997).
Ying, S. et al.Expression of IL-4 and IL-5 mRNA and protein product by CD4+ and CD8+ T cells, eosinophils and mast cells in bronchial biopsies obtained from atopic and non-atopic (intrinsic asthmatics). J. Immunol. 158, 3539–3544 (1997).
Maestrelli, P. et al. Cytokines in the airway mucosa of subjects with asthma induced by toluene diisocyanate. Am. J. Respir. Crit. Care Med. 151, 607–612 (1995).
Holgate, S. The inflammation-repair cycle in asthma: the pivotal role of the airway epithelium. Clin. Exp. Allergy 28 S5, 97– 103 (1998).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Holt, P., Macaubas, C., Stumbles, P. et al. The role of allergy in the development of asthma. Nature 402 (Suppl 6760), 12–17 (1999). https://doi.org/10.1038/35037009
Issue Date:
DOI: https://doi.org/10.1038/35037009
This article is cited by
-
Antibody blockade of Dectin-2 suppresses house dust mite-induced Th2 cytokine production in dendritic cell- and monocyte-depleted peripheral blood mononuclear cell co-cultures from asthma patients
Journal of Biomedical Science (2019)
-
The Microbiome and the Pathophysiology of Asthma
Respiratory Research (2016)
-
An Overlook to the Characteristics and Roles Played by Eotaxin Network in the Pathophysiology of Food Allergies: Allergic Asthma and Atopic Dermatitis
Inflammation (2016)
-
Nanoparticle conjugation enhances the immunomodulatory effects of intranasally delivered CpG in house dust mite-allergic mice
Scientific Reports (2015)
-
Toll-Like Receptors Link Atopic March to the Hygiene Hypothesis
Journal of Investigative Dermatology (2013)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.