Abstract
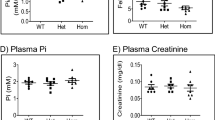
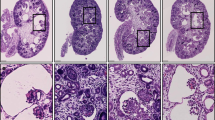
Dent's disease is an X-linked disorder associated with the urinary loss of low-molecular-weight proteins, phosphate and calcium, which often leads to kidney stones1,2. It is caused by mutations3 in ClC-5, a renal chloride channel4,5 that is expressed in endosomes of the proximal tubule6,7. Here we show that disruption of the mouse clcn5 gene causes proteinuria by strongly reducing apical proximal tubular endocytosis. Both receptor-mediated and fluid-phase endocytosis are affected, and the internalization of the apical transporters NaPi-2 and NHE3 is slowed. At steady state, however, both proteins are redistributed from the plasma membrane to intracellular vesicles. This may be caused by an increased stimulation of luminal parathyroid hormone (PTH) receptors owing to the observed decreased tubular endocytosis of PTH. The rise in luminal PTH concentration should also stimulate the hydroxylation of 25(OH) vitamin D3 to the active hormone. However, this is counteracted by a urinary loss of the precursor 25(OH) vitamin D3. The balance between these opposing effects, both of which are secondary to the defect in proximal tubular endocytosis, probably determines whether there will be hypercalciuria and kidney stones.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Wrong, O. M., Norden, A. G. & Feest, T. G. Dent's disease; a familial proximal renal tubular syndrome with low-molecular-weight proteinuria, hypercalciuria, nephrocalcinosis, metabolic bone disease, progressive renal failure and a marked male predominance. Q. J. Med. 87, 473–493 (1994).
Scheinman, S. J. X-linked hypercalciuric nephrolithiasis: clinical syndromes and chloride channel mutations. Kidney Int. 53, 3– 17 (1998).
Lloyd, S. E. et al. A common molecular basis for three inherited kidney stone diseases. Nature 379, 445– 449 (1996).
Fisher, S. E. et al. Isolation and partial characterization of a chloride channel gene which is expressed in kidney and is a candidate for Dent's disease (an X-linked hereditary nephrolithiasis). Hum. Mol. Genet. 3, 2053–2059 (1994).
Steinmeyer, K., Schwappach, B., Bens, M., Vandewalle, A. & Jentsch, T. J. Cloning and functional expression of rat CLC-5, a chloride channel related to kidney disease. J. Biol. Chem. 270, 31172–31177 (1995).
Günther, W., Lüchow, A., Cluzeaud, F., Vandewalle, A. & Jentsch, T. J. ClC-5, the chloride channel mutated in Dent's disease, colocalizes with the proton pump in endocytotically active kidney cells. Proc. Natl Acad. Sci. USA 95, 8075–8080 (1998).
Devuyst, O., Christie, P. T., Courtoy, P. J., Beauwens, R. & Thakker, R. V. Intra-renal and subcellular distribution of the human chloride channel, CLC-5, reveals a pathophysiological basis for Dent's disease. Hum. Mol. Genet. 8, 247– 257 (1999).
Jentsch, T. J., Friedrich, T., Schriever, A. & Yamada, H. The CLC chloride channel family. Pflügers Arch. 437, 783–795 (1999).
Friedrich, T., Breiderhoff, T. & Jentsch, T. J. Mutational analysis demonstrates that ClC-4 and ClC-5 directly mediate plasma membrane currents. J. Biol. Chem. 274, 896–902 (1999).
Luyckx, V. A., Leclercq, B., Dowland, L. K. & Yu, A. S. Diet-dependent hypercalciuria in transgenic mice with reduced CLC5 chloride channel expression. Proc. Natl Acad. Sci. USA 96, 12174–12179 (1999).
Morimoto, T. et al. Mutations in CLCN5 chloride channel in Japanese patients with low molecular weight proteinuria. J. Am. Soc. Nephrol. 9, 811–818 (1998).
Leheste, J. R. et al. Megalin knockout mice as an animal model of low molecular weight proteinuria. Am. J. Pathol. 155, 1361–1370 (1999).
Orlando, R. A. et al. Megalin is an endocytic receptor for insulin. J. Am. Soc. Nephrol. 9, 1759–1766 (1998).
Nykjaer, A. et al. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell 96, 507–515 (1999).
Christensen, E. I. et al. Evidence for an essential role of megalin in transepithelial transport of retinol. J. Am. Soc. Nephrol. 10, 685–695 (1999).
Sakamoto, H. et al. Cellular and subcellular immunolocalization of ClC-5 channel in mouse kidney: colocalization with H+-ATPase. Am. J. Physiol. 277, F957–F965 (1999).
Beck, L. et al. Targeted inactivation of Npt2 in mice leads to severe renal phosphate wasting, hypercalciuria, and skeletal abnormalities. Proc. Natl Acad. Sci. USA 95, 5372–5377 (1998).
Murer, H. et al. Posttranscriptional regulation of the proximal tubule NaPi-II transporter in response to PTH and dietary Pi. Am. J. Physiol. 277, F676–F684 ( 1999).
Biemesderfer, D. et al. Monoclonal antibodies for high-resolution localization of NHE3 in adult and neonatal rat kidney. Am. J. Physiol. 273, F289–F299 (1997).
D'Souza, S. et al. The epithelial sodium-hydrogen antiporter Na+/H+ exchanger 3 accumulates and is functional in recycling endosomes. J. Biol. Chem. 273, 2035– 2043 (1998).
Traebert, M., Roth, J., Biber, J., Murer, H. & Kaissling, B. Internalization of proximal tubular type II Na-Pi cotransporter by PTH: immunogold electron microscopy. Am. J. Physiol. 278, F148–F154 (2000).
Zhang, Y. et al. In vivo PTH provokes apical NHE3 and NaPi2 redistribution and Na-K-ATPase inhibition. Am. J. Physiol. 276, F711-F719 (1999).
Custer, M., Lotscher, M., Biber, J., Murer, H. & Kaissling, B. Expression of Na-Pi cotransport in rat kidney: localization by RT-PCR and immunohistochemistry. Am. J. Physiol. 266, F767–F774 (1994).
Bosio, M., Bianchi, M. L., Lloyd, S. E. & Thakker, R. V. A familial syndrome due to Arg648Stop mutation in the X-linked renal chloride channel gene. Pediatr. Nephrol. 13, 278– 283 (1999).
Kaufmann, M. et al. Apical and basolateral parathyroid hormone receptors in rat renal cortical membranes. Endocrinology 134, 1173–1178 (1994).
Traebert, M., Völkl, H., Biber, J., Murer, H. & Kaissling, B. Luminal and contraluminal action of 1-34 and 3-34 PTH peptides on renal type IIa Na-Pi cotransporter. Am. J. Physiol. 278, F792–F798 (2000).
Hilpert, J. et al. Megalin antagonizes activation of the parathyroid hormone receptor. J. Biol. Chem. 274, 5620– 5625 (1999).
Gekle, M. et al. Inhibition of Na+-H+ exchange impairs receptor-mediated albumin endocytosis in renal proximal tubule-derived epithelial cells from opossum. J. Physiol. (Lond.) 520, 709–721 (1999).
Schultheis, P. J. et al. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nature Genet. 19, 282–285 ( 1998).
Zheng, G. et al. Organ distribution in rats of two members of the low-density lipoprotein receptor gene family, gp330 and LRP/alpa 2MR, and the receptor-associated protein (RAP). J. Histochem. Cytochem. 42, 531–542 (1994).
Acknowledgements
We thank G. Boia for technical assistance, P. Gruss for MPI2 ES cells, H. Murer and J. Biber for the NaPi-2 antibody, R. McCluskey for the megalin antibody, S. Gluck and D. K. Stone for proton pump antibodies, B. Blaner for the antibody against retinol binding protein and M. Haddad for chemical analysis of serum and urine samples. This work was supported by grants from the Deutsche Forschungsgemeinschaft, the Fonds der Chemischen Industrie, and the Louis-Jeantet Prize for medicine to T.J.J.
Author information
Authors and Affiliations
Supplementary information
Rights and permissions
About this article
Cite this article
Piwon, N., Günther, W., Schwake, M. et al. ClC-5 Cl--channel disruption impairs endocytosis in a mouse model for Dent's disease. Nature 408, 369–373 (2000). https://doi.org/10.1038/35042597
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35042597
This article is cited by
-
Genetica dei rachitismi ereditari da alterazione dell’omeostasi del fosfato
L'Endocrinologo (2024)
-
A novel likely pathogenic CLCN5 variant in Dent’s disease
BMC Nephrology (2023)
-
Proton-gated anion transport governs macropinosome shrinkage
Nature Cell Biology (2022)
-
Glomerular podocyte dysfunction in inherited renal tubular disease
World Journal of Pediatrics (2021)
-
Genetics and phenotypic heterogeneity of Dent disease: the dark side of the moon
Human Genetics (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.