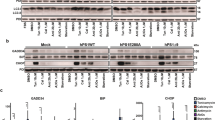
Abstract
Presenilin 1 (PS1), a polytopic membrane protein, has a critical role in the trafficking and proteolysis of a selected set of transmembrane proteins. The vast majority of individuals affected with early onset familial Alzheimer's disease (FAD) carry missense mutations in PS1. Two studies have suggested that loss of PS1 function, or expression of FAD-linked PS1 variants, compromises the mammalian unfolded-protein response (UPR), and we sought to evaluate the potential role of PS1 in the mammalian UPR. Here we show that that neither the endoplasmic reticulum (ER) stress-induced accumulation of BiP and CHOP messenger RNA, nor the activation of ER stress kinases IRE1α and PERK, is compromised in cells lacking both PS1 and PS2 or in cells expressing FAD-linked PS1 variants. We also show that the levels of BiP are not significantly different in the brains of individuals with sporadic Alzheimer's disease or PS1-mediated FAD to levels in control brains. Our findings provide evidence that neither loss of PS1 and PS2 function, nor expression of PS1 variants, has a discernable impact on ER stress-mediated induction of the several established `readouts' of the UPR pathway.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Glenner, G. G. & Wong, C. W. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Comm. 120, 885–890 (1984).
Masters, C. L. et al. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc. Natl Acad. Sci. USA 82, 4245–4249 (1985).
Sherrington, R. et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer's disease. Nature 375, 754–760 (1995).
Levy-Lahad, E. et al. Candidate gene for the chromosome 1 familial Alzheimer's disease locus. Science 269, 973– 977 (1995).
Rogaev, E. I. et al. Familial Alzheimer's disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer's disease type 3 gene. Nature 376, 775–778 ( 1995).
Thinakaran, G. et al. Endoproteolysis of presenilin 1 and accumulation of processed derivatives in vivo. Neuron 17, 181 –190 (1996).
De Strooper, B. et al. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature 391, 387 –390 (1998).
Naruse, S. et al. Effects of PS1 deficiency on membrane protein trafficking in neurons. Neuron 21, 1213– 1221 (1998).
De Strooper, B. et al. A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature 398, 518–522 (1999).
Scheuner, D. et al. Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer's disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer's disease. Nature Med. 2, 864–870 ( 1996).
Borchelt, D.R. et al. Familial Alzheimer's disease-linked presenilin 1 variants elevate Abeta1-42/1-40 ratio in vitro and in vivo. Neuron 17, 1005–1013 ( 1996).
Tomita, T. et al. The presenilin 2 mutation (N141I) linked to familial Alzheimer disease (Volga German families) increases the secretion of amyloid beta protein ending at the 42nd (or 43rd) residue. Proc. Natl Acad. Sci. USA 94, 2025–2030 ( 1997).
Thinakaran, G. The role of presenilins in Alzheimer's disease. J. Clin. Invest. 104, 1321–1327 ( 1999).
Niwa, M., Sidrauski, C., Kaufman, R. J. & Walter, P. A role for presenilin-1 in nuclear accumulation of Ire1 fragments and induction of the mammalian unfolded protein response. Cell 99 , 691–702 (1999).
Katayama, T. et al. Presenilin-1 mutations downregulate the signalling pathway of the unfolded-protein response. Nature Cell Biol. 1, 479–485 (1999).
Kaufman, R. J. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 13, 1211–1233 ( 1999).
Pahl, H. L. Signal transduction from the endoplasmic reticulum to the cell nucleus. Physiol. Rev. 79, 683–701 (1999).
Shamu, C. E. & Walter, P. Oligomerization and phosphorylation of the Ire1p kinase during intracellular signaling from the endoplasmic reticulum to the nucleus. EMBO J. 15, 3028–3039 (1996).
Sidrauski, C. & Walter, P. The transmembrane kinase Ire1p is a site-specific endonuclease that initiates mRNA splicing in the unfolded protein response. Cell 90, 1031 –1039 (1997).
Sidrauski, C., Chapman, R. & Walter, P. The unfolded protein response: an intracellular signalling pathway with many surprising features. Trends Cell Biol. 8, 245–249 ( 1998).
Bertolotti, A., Zhang, Y., Hendershot, L. M., Harding, H. P. & Ron, D. Dynamic interaction of BiP and ER stress transducers in the unfolded- protein response. Nature Cell Biol. 2, 326–332 (2000).
Price, B. D. & Calderwood, S. K. Gadd45 and Gadd153 messenger RNA levels are increased during hypoxia and after exposure of cells to agents which elevate the levels of the glucose-regulated proteins. Cancer Res. 52, 3814–3817 (1992).
Wang, X. Z. et al. Signals from the stressed endoplasmic reticulum induce C/EBP-homologous protein (CHOP/GADD153). Mol. Cell. Biol. 16, 4273–4280 (1996).
Harding, H. P., Zhang, Y., Bertolotti, A., Zeng, H. & Ron, D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol. Cell 5, 897–904 ( 2000).
Shi, Y. et al. Identification and characterization of pancreatic eukaryotic initiation factor 2 alpha-subunit kinase, PEK, involved in translational control. Mol. Cell. Biol. 18, 7499–7509 (1998).
Harding, H. P., Zhang, Y. & Ron, D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397, 271– 274 (1999).
Thinakaran, G. et al. Evidence that levels of presenilins (PS1 and PS2) are coordinately regulated by competition for limiting cellular factors. J. Biol. Chem. 272, 28415–28422 ( 1997).
Suzuki, N. et al. An increased percentage of long amyloid beta protein secreted by familial amyloid beta protein precursor (beta APP717) mutants. Science 264, 1336–1340 ( 1994).
Li, W.W., Alexandre, S., Cao, X. & Lee, A.S. Transactivation of the grp78 promoter by Ca2+ depletion. A comparative analysis with A23187 and the endoplasmic reticulum Ca(2+)-ATPase inhibitor thapsigargin . J. Biol. Chem. 268, 12003– 12009 (1993).
Hendriks, L. et al. Processing of presenilin 1 in brains of patients with Alzheimer's disease and controls. NeuroReport 8, 1717 –1721 (1997).
Borchelt, D. R. et al. A vector for expressing foreign genes in the brains and hearts of transgenic mice. Genet. Anal. 13, 159 –163 (1996).
Borchelt, D. R. et al. Accelerated amyloid deposition in the brains of transgenic mice coexpressing mutant presenilin 1 and amyloid precursor proteins. Neuron 19, 939–945 ( 1997).
Mattson, M. P., Guo, Q., Furukawa, K. & Pedersen, W. A. Presenilins, the endoplasmic reticulum, and neuronal apoptosis in Alzheimer's disease. J. Neurochem. 70, 1– 14 (1998).
Wong, P. C. et al. Presenilin 1 is required for Notch1 and DII1 expression in the paraxial mesoderm. Nature 387, 288– 292 (1997).
Donoviel, D. B. et al. Mice lacking both presenilin genes exhibit early embryonic patterning defects. Genes Dev. 13, 2801– 2810 (1999).
Hogan, B., Beddington, R., Costantini, F. & Lacey, E. Manipulating the Mouse Embryo: a Laboratory Manual (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, 1994).
Thinakaran, G., Teplow, D. B., Siman, R., Greenberg, B. & Sisodia, S. S. Metabolism of the “Swedish” amyloid precursor protein variant in neuro2a (N2a) cells. Evidence that cleavage at the “β-secretase” site occurs in the golgi apparatus. J. Biol. Chem. 271, 9390–9397 (1996).
Saura, C. A. et al. The non-conserved hydrophilic loop domain of presenilin (PS) is neither required for PS endoproteolysis nor enhanced Aβ42 production mediated by familial Alzheimer's disease-linked PS variants. J. Biol. Chem. 275, 17136–17142 (2000).
Cruts, M. et al. Molecular genetic analysis of familial early-onset Alzheimer's disease linked to chromosome 14q24.3. Hum. Mol. Genet. 4, 2363–2371 (1995).
Thinakaran, G. et al. Stable association of presenilin derivatives and absence of presenilin interactions with APP. Neurobiol. Dis. 4 , 438–453 (1998).
Li, M. et al. ATF6 as a transcription activator of the endoplasmic reticulum stress element: thapsigargin stress-induced changes and synergistic interactions with NF-Y and YY1. Mol. Cell. Biol. 20, 5096–5106 (2000).
Acknowledgements
This work was supported by NIH 1PO1 grant AG14248 (S.S.S.), NIEHS grant ES08681 (D.R), NIH, NCI grant CA27607 (A.S.L), the Alzheimer's Association (G.T), the Adler Foundation (G.T. and J.Y.L), and by an award to the University of Chicago's Division of Biological Sciences under the Research Resources Program for Medical Schools of the Howard Hughes Medical Institute (G.T.). N.S. and F.U are supported by a research fellowship from the Japan Society for the Promotion of Science and grants-in-aid from the Ministry of Education, Science, Culture and Sports, Japan. We thank C. A. Saura, T. Tomita and T. Iwatsubo for Aβ analysis; D. R. Borchelt for transgenic mouse tissue; J. Troncoso for control and sporadic AD brain tissue; and L. Hendriks and C. V. Broeckhoven for FAD brain tissue.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Figure S1
Semi-quantitative RT-PCR analysis of BiP and CHOP mRNA levels in PS1-/- and PS1+/- fibroblasts. (PDF 224 kb)
Figure S2 Activation of the UPR is unimpaired by the expression of a loss of function PS1 mutant.
Figure S3 Kinetic analysis of BiP and CHOP mRNA in N2a cell lines expressing Wt PS1 or PS1 D385A mutant.
Figure S4 Coomassie blue staining of brain homogenates of controls, FAD patients and sporadic AD patients.
Rights and permissions
About this article
Cite this article
Sato, N., Urano, F., Yoon Leem, J. et al. Upregulation of BiP and CHOP by the unfolded-protein response is independent of presenilin expression. Nat Cell Biol 2, 863–870 (2000). https://doi.org/10.1038/35046500
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/35046500
This article is cited by
-
Human skin specific long noncoding RNA HOXC13-AS regulates epidermal differentiation by interfering with Golgi-ER retrograde transport
Cell Death & Differentiation (2023)
-
Arginine regulates HSPA5/BiP translation through ribosome pausing in triple-negative breast cancer cells
British Journal of Cancer (2023)
-
Insights into the role of paraoxonase 2 in human pathophysiology
Journal of Biosciences (2022)
-
Susceptibility to cellular stress in PS1 mutant N2a cells is associated with mitochondrial defects and altered calcium homeostasis
Scientific Reports (2020)
-
Endoplasmic reticulum stress and inflammation in the central nervous system
Molecular Neurodegeneration (2017)