Abstract
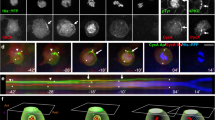
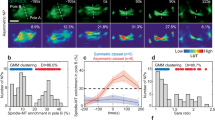
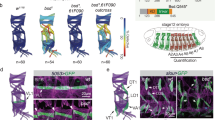
Asymmetric partitioning of cell-fate determinants during development requires coordinating the positioning of these determinants with orientation of the mitotic spindle. In the Drosophila peripheral nervous system, sensory organ progenitor cells (SOPs) undergo several rounds of division to produce five cells that give rise to a complete sensory organ. Here we have observed the asymmetric divisions that give rise to these cells in the developing pupae using green fluorescent protein fusion proteins. We find that spindle orientation and determinant localization are tightly coordinated at each division. Furthermore, we find that two types of asymmetric divisions exist within the sensory organ precursor cell lineage: the anterior–posterior pI cell-type division, where the spindle remains symmetric throughout mitosis, and the strikingly neuroblast-like apical–basal division of the pIIb cell, where the spindle exhibits a strong asymmetry at anaphase. In both these divisions, the spindle reorientates to position itself perpendicular to the region of the cortex containing the determinant. On the basis of these observations, we propose that two distinct mechanisms for controlling asymmetric cell divisions occur within the same lineage in the developing peripheral nervous system in Drosophila.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Horvitz, H. & Herskowitz, I. Mechanisms of asymmetric cell division: two B's or not two B's, that is the question. Cell 68, 237–255 ( 1992).
Jan, Y. & Jan, L. Polarity in cell division: what frames thy fearful asymmetry. Cell 100, 599– 602 (2000).
Takizawa, P., Sil, A., Swedlow, J., Herskowitz, I. & Vale, R. Actin-dependent localization of an RNA encoding a cell fate determinant in yeast. Nature 389 , 90–93 (1997).
Long, R. et al. Mating type switching in yeast controlled by asymmetric localization of Ash1 mRNA. Science 277, 383– 387 (1997).
Lee, L. et al. Positioning of the mitotic spindle by a cortical-microtubule capture mechanism. Science 287, 2260– 2262 (2000).
Carminati, J. & Stearns, T. Microtubules orient the mitotic spindle in yeast through dynein-dependent interactions with the cell cortex. J. Cell Biol. 138, 629– 641 (1997).
Shaw, S., Yeh, E., Maddox, P., Salmon, E. & Bloom, K. Astral microtubule dynamics in yeast: a microtubule-based searching mechanism for spindle orientation and nuclear migration into the bud. J. Cell Biol. 139, 985–994 (1997).
Stearns, T. Motoring to the finish: kinesin and dynein work together to orient the yeast mitotic spindle. J. Cell Biol. 138, 957– 960 (1997).
Korinek, W., Copeland, M., Chaudhuri, A. & Chant, J. Molecular linkage underlying microtubule orientation toward cortical sites in yeast. Science 287, 2257– 2259 (2000).
Hyman, A. & White, J. Determination of cell division axes in the early embryogenesis of Caenorhabditis elegans. J. Cell Biol. 105, 2123–2135 (1987).
Rose, L. & Kemphues, K. Early patterning of the C. elegans embryo. Annu. Rev. Genet. 32, 521–545 (1998).
Gho, M., Bellaiche, Y. & Schweisguth, F. Revisiting the Drosophila microchaete lineage: a novel intrinsically asymmetric cell division generates a glial cell. Development 126, 3573–3584 (1999).
Kaltschmidt, J., Davidson, C., Brown, N. & Brand, A. Rotation and asymmetry of the mitotic spindle direct asymmetric cell division in the developing central nervous system. Nat ure Cell Biol. 2, 7–12 (2000).
Lu, B., Ackerman, L., Jan, L. & Jan, Y. Modes of protein movement that lead to the asymmetric localization of Partner of Numb during Drosophila neuroblast division. Mol. Cell 4, 883–891 (1999).
Bate, M. in Handbook of Sensory Physiology Vol. IX (ed. Jakobson, M.) 1–53 (Springer, Berlin, 1978).
Gomez, M. & Bate, M. Segregation of myogenic lineages in Drosophila requires numb. Development 124, 4857–4866 ( 1997).
Buescher, M. et al. Binary sibling neuronal cell fate decisions in the Drosophila embryonic central nervous system are nonstochastic and require inscuteable -mediated asymmetry of ganglion mother cells. Genes Dev. 12, 1858–1870 (1998).
Kraut, R., Chia, W., Jan, L., Jan, Y. & Knoblich, J. Role of inscuteable in orienting asymmetric cell divisions in Drosophila. Nature 383, 50–55 (1996).
Carmena, A., Murugasu-Oei, B., Menon, D., Jimenez, F. & Chia, W. inscuteable and numb mediate asymmetric muscle progenitor cell divisions during Drosophila myogenesis. Genes & Dev. 12, 304 –315 (1998).
Knoblich, J., Jan, L. & Jan, Y. Asymmetric segregation of Numb and Prospero during cell division. Nature 377, 624–630 ( 1995).
Spana, E., Kopczynski, C., Goodman, C. & Doe, C. Asymmetric localization of Numb autonomously determines sibling neuron identity in Drosophila CNS. Development 121, 3489–3494 (1995).
Rhyu, M., Jan, L. & Jan, Y. Asymmetric distribution of Numb protein during division of the sensory organ precursor cell confers distinct fates to daughter cells. Cell 76, 477–491 (1994).
Gho, M. & Schweisguth, F. Frizzled signalling controls orientation of asymmetric sense organ precursor cell divisions in Drosophila . Nature 393, 178–181 (1998).
Lu, B., Usui, T., Uemura, T., Jan, L. & Jan, Y. Flamingo controls the planar polarity of sensory bristles and asymmetric division of sensory organ precursors in Drosophila. Curr. Biol. 9, 1247–1250 (1999).
Schober, M., Schaefer, M. & Knoblich, J. Bazooka recruits Inscuteable to orient asymmetric cell divisions in Drosophila neuroblasts. Nature 402, 548–551 (1999).
Kraut, R. & Campos-Ortega, J. inscuteable , a neural precursor gene of Drosophila, encodes a candidate for a cytoskeleton adaptor protein. Dev. Biol. 174, 65–81 (1996).
Kuchinke, U., Grawe, F. & Knust, E. Control of spindle orientation in Drosophila by the Par-3-related PDZ-domain protein Bazooka. Curr. Biol. 8, 1357–1365 ( 1998).
Yu, F., Morin, X., Cai, Y., Yang, X. & Chia, W. Analysis of partner of inscuteable, a novel player of Drosophila asymmetric divisions, reveals two distinct steps in Inscuteable apical localization. Cell 100, 399– 409 (2000).
Wodarz, A., Ramrath, A., Kuchinke, U. & Knust, E. Bazooka provides an apical cue for Inscuteable localization in Drosophila neuroblasts. Nature 402, 544– 547 (1999).
Bonnaccorsi, S., Giansanti, M. & Gatti, M. Spindle assembly in Drosophila neuroblasts and ganglion mother cells. Nature Cell Biol. 2, 54–56 (2000).
Lu, B., Rothenberg, M., Jan, L. & Jan, Y. Partner of Numb colocalizes with Numb during mitosis and directs Numb asymmetric localization in Drosophila neural and muscle progenitors. Cell 95, 225–235 (1998).
Brand, A. & Perrimon, N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401–415 (1993).
Chiba, A. Personal communication to Flybase. http://flybase.bio.indiana.edu/.bin/fbpcq.html?FBrf0102855 (1998).
Brand, A. GFP in Drosophila. Trends Genet. 11, 324–325 (1995).
Edwards, K., Demsky, M., Montague, R., Weymouth, N. & Kiehart, D. GFP–Moesin illuminates actin cytoskeleton dynamics in living tissue and demonstrates cell shape changes during morphogenesis in Drosophila. Dev. Biol. 191, 103–117 (1997).
Li, P., Yang, X., Wasser, M., Cai, Y. & Chia, W. Inscuteable and Staufen mediate asymmetric localization and segregation of prospero RNA during Drosophila neuroblast cell divisions. Cell 90, 437– 447 (1997).
Hassan, B. et al. skittles, a Drosophila phosphatidylinositol 4-phosphate 5-kinase, is required for cell viability, germline development and bristle morphology, but not for neurotransmitter release. Genetics 150, 1527–1537 (1998).
Ramírez-Weber, F. & Kornberg, T. Cytonemes: cellular processes that project to the principal signaling center in Drosophila imaginal discs. Cell 97, 599– 607 (1999).
Schuldt, A. et al. Miranda mediates asymmetric protein and RNA localization in the developing nervous system. Genes & Dev. 12, 1847–1857 (1998).
Shen, C.-P., Jan, L. & Jan, Y. Miranda is required for the asymmetric localization of prospero during mitosis in Drosophila. Cell 90, 449– 458 (1997).
Shen, C.-P. et al. Miranda as a multidomain adapter linking apically localized Inscuteable and basally localized Staufen and Prospero during asymmetric cell division in Drosophila. Genes Dev. 12, 1837–1846 (1998).
Fuerstenberg, S., Peng, C., Alvarez-Ortiz, P., Hor, T. & Doe, C. Identification of Miranda protein domains regulating asymmetric cortical localization, cargo binding, and cortical release. Mol. Cell. Neurosci. 12, 325–339 (1998).
Reddy, G. & Rodrigues, V. Sibling cell fate in the Drosophila adult external sense organ lineage is specified by Prospero function, which is regulated by Numb and Notch. Development 126, 2083–2092 ( 1999).
Manning, L. & Doe, C. Prospero distinguishes sibling cell fate without asymmetric localization in the Drosophila adult external sense organ lineage. Development 126 , 2063–2071 (1999).
Hyman, A. Centrosome movement in the early divisions of Caenorhabditis elegans: a cortical site determining centrosome position. J. Cell Biol. 109, 1185–1193 ( 1989).
Lee, T. & Luo, L. Mosaic analysis with a repressible cell marker for studies of gene function in neural morphogenesis. Neuron 22, 451–461 ( 1999).
Lutz, D. & Inoue, S. Techniques for observing living gametes and embryos. Methods Cell Biol. 27, 89–110 (1986).
Wang, S., Younger-Shepherd, S., Jan, L. & Jan, Y. Only a subset of the binary cell fate decisions mediated by Numb/Notch signaling in Drosophila sensory organ lineage requires Supressor of Hairless . Development 124, 4435– 4464 (1997).
Acknowledgements
We thank Y. M. Chan for critical reading of the manuscript, B. Lu for Sca–Gal4 UAS–Pon–GFP/Cyo and UAS–Numb–GFP flies. We thank F. Schweisguth, D. Kiehart, W. Chia and the Bloomington Stock Center for flies and reagents. We also thank members of the Jan lab for helpful discussions. F.R. is supported by the NIH Neuroscience Training Grant and the Human Frontiers Science Program (HFSP) postdoctoral fellowship. L.Y.J. and Y.N.J. are investigators at the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Movie 1a
(See Fig. 4a) Dorsal view of pupa 16^h after formation. The spindle seesaws while the Pon crescent accumulates at the anterior cortex during SOP cell mitosis. When the Pon crescent completes its accumulation the spindle stops moving and undergoes anaphase. Microtubules were labelled with tau-GFP, Pon with Pon-GFP. Frames were recorded every 40^s. Anterior is to the bottom right. (AVI 1504 kb)
Movie 2b (See Fig. 5d)
Dorsal view of pupa ~16^h after formation. The spindle seesaws while the Pon crescent accumulates at the anterior cortex during SOP cell mitosis in an fz mutant. When the Pon crescent completes its accumulation the spindle stops moving and undergoes anaphase. Microtubules were labelled with tau-GFP, Pon with Pon-GFP. Frames were recorded every 15^s. Anterior is to the bottom right. (AVI 823 kb)
Movie 2b (See Fig. 5d)
As Movie 2a. (AVI 823 kb)
Movie 3a
(See Fig. 5e) Dorsal view of pupa ~16^h after formation. In 10% of fz-mutant SOPs the mitotic spindle remains parallel to the Pon-GFP crescent (SOP lower right). In 90% of cases the spindle is orientated perpendicular to the Pon crescent (SOP upper left). At anaphase, in both cases, the Pon-GFP crescent segregates to one of the two daughter cells. Microtubules were labeled with tau-GFP, Pon with Pon-GFP. Frames were recorded every 15^s. Anterior is to the upper right. (AVI 3269 kb)
Rights and permissions
About this article
Cite this article
Roegiers, F., Younger-Shepherd, S., Jan, L. et al. Two types of asymmetric divisions in the Drosophila sensory organ precursor cell lineage. Nat Cell Biol 3, 58–67 (2001). https://doi.org/10.1038/35050568
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/35050568
This article is cited by
-
Cortical Cyclin A controls spindle orientation during asymmetric cell divisions in Drosophila
Nature Communications (2022)
-
Kinetochore-localized PP1–Sds22 couples chromosome segregation to polar relaxation
Nature (2015)
-
Mechanisms of asymmetric cell division: flies and worms pave the way
Nature Reviews Molecular Cell Biology (2008)
-
aPKC-mediated phosphorylation regulates asymmetric membrane localization of the cell fate determinant Numb
The EMBO Journal (2007)
-
A protocol for mosaic analysis with a repressible cell marker (MARCM) in Drosophila
Nature Protocols (2006)