Abstract
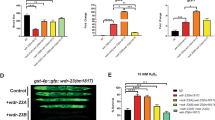
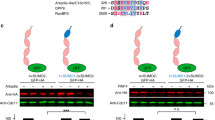
SCF ubiquitin ligases are composed of Skp1, Cdc53, Hrt1 and one member of a large family of substrate receptors known as F-box proteins (FBPs). Here we report the identification, using sequential rounds of epitope tagging, affinity purification and mass spectrometry, of 16 Skp1 and Cdc53-associated proteins in budding yeast, including all components of SCF, 9 FBPs, Yjr033 (Rav1) and Ydr202 (Rav2). Rav1, Rav2 and Skp1 form a complex that we have named `regulator of the (H+)-ATPase of the vacuolar and endosomal membranes' (RAVE), which associates with the V1 domain of the vacuolar membrane (H+)-ATPase (V-ATPase). V-ATPases are conserved throughout eukaryotes, and have been implicated in tumour metastasis and multidrug resistance, and here we show that RAVE promotes glucose-triggered assembly of the V-ATPase holoenzyme. Previous systematic genome-wide two-hybrid screens yielded 17 proteins that interact with Skp1 and Cdc53, only 3 of which overlap with those reported here. Thus, our results provide a distinct view of the interactions that link proteins into a comprehensive cellular network.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Deshaies, R. J. SCF and Cullin/Ring H2-based ubiquitin ligases. Annu. Rev. Cell Dev. Biol. 15, 435–467 (1999).
Tan, P. et al. Recruitment of a ROC1–CUL1 ubiquitin ligase by Skp1 and HOS to catalyze the ubiquitination of IκBα. Mol. Cell 3, 527–533 (1999).
Ohta, T., Michel, J. J., Schottelius, A. J. & Xiong, Y. ROC1, a homolog of APC11, represents a family of cullin partners with an associated ubiquitin ligase activity. Mol. Cell 3, 535–541 (1999).
Kamura, T. et al. Rbx1, a component of the VHL tumor suppressor complex and SCF ubiquitin ligase. Science 284, 657–661 (1999).
Seol, J. H. et al. Cdc53/cullin and the essential Hrt1 RING-H2 subunit of SCF define a ubiquitin ligase module that activates the E2 enzyme Cdc34. Genes Dev. 13, 1614–1626 (1999).
Skowyra, D., Craig, K. L., Tyers, M., Elledge, S. J. & Harper, J. W. F-box proteins are receptors that recruit phosphorylated substrates to the SCF ubiquitin-ligase complex. Cell 91, 209–219 (1997).
Patton, E. E., Willems, A. R. & Tyers, M. Combinatorial control in ubiquitin-dependent proteolysis: don't Skp the F-box hypothesis. Trends Genet. 14, 236–243 (1998).
Patton, E. E. et al. Cdc53 is a scaffold protein for multiple Cdc34/Skp1/F-box proteincomplexes that regulate cell division and methionine biosynthesis in yeast. Genes Dev. 12, 692–705 (1998).
Bartel, P. L., Roecklein, J. A., SenGupta, D. & Fields, S. A protein linkage map of Escherichia coli bacteriophage T7. Nature Genet. 12, 72–77 (1996).
Fromont-Racine, M., Rain, J. C. & Legrain, P. Toward a functional analysis of the yeast genome through exhaustive two-hybrid screens. Nature Genet. 16, 277–282 (1997).
Stevens, T. H. & Forgac, M. Structure, function and regulation of the vacuolar (H+)-ATPase. Annu. Rev. Cell Dev. Biol. 13, 779–808 (1997).
Forgac, M. Structure and properties of the vacuolar (H+)-ATPases. J. Biol. Chem. 274, 12951–12954 (1999).
Parra, K. J. & Kane, P. M. Reversible association between the V1 and V0 domains of yeast vacuolar H+-ATPase is an unconventional glucose-induced effect. Mol. Cell. Biol. 18, 7064–7074 (1998).
Zhang, J. W., Parra, K. J., Liu, J. & Kane, P. M. Characterization of a temperature-sensitive yeast vacuolar ATPase mutant with defects in actin distribution and bud morphology. J. Biol. Chem. 273, 18470–18480 (1998).
Wiederkehr, A., Avaro, S., Prescianotto-Baschong, C., Haguenauer-Tsapis, R. & Riezman, H. The F-box protein Rcy1p is involved in endocytic membrane traffic and recycling out of an early endosome in Saccharomyces cerevisiae. J. Cell. Biol. 149, 397–410 (2000).
Winzeler, E. A. et al. Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science 285, 901–906 (1999).
Spellman, P. T. et al. Comprehensive identification of cell cycle-regulated genes of the yeast Saccharomyces cerevisiae by microarray hybridization. Mol. Biol. Cell 9, 3273–3297 (1998).
Uetz, P. et al. A comprehensive analysis of protein–protein interactions in Saccharomyces cerevisiae. Nature 403, 623–627 (2000).
Marcotte, E. M. et al. Detecting protein function and protein–protein interactions from genome sequences. Science 285, 751–753 (1999).
Kaplan, K. B., Hyman, A. A. & Sorger, P. K. Regulating the yeast kinetochore by ubiquitin-dependent degradation and Skp1p-mediated phosphorylation. Cell 91, 491–500 (1997).
Galan, J. M. et al. Skp1p and the F-box protein Rcy1p form a non-SCF complex involved in recycling of the SNARE Snc1p in yeast. Mol. Cell. Biol. (in press).
Foury, F. The 31-kDa polypeptide is an essential subunit of the vacuolar ATPase in Saccharomyces cerevisiae. J. Biol. Chem. 265, 18554–18560 (1990).
Weisman, L. S., Bacallao, R. & Wickner, W. Multiple methods of visualizing the yeast vacuole permit evaluation of its morphology and inheritance during the cell cycle. J. Cell. Biol. 105, 1539–1547 (1987).
Manolson, M. F. et al. The VPH1 gene encodes a 95-kDa integral membrane polypeptide required for in vivo assembly and activity of the yeast vacuolar H(+)-ATPase. J. Biol. Chem. 267, 14294–14303 (1992).
Rigaut, G. et al. A generic protein purification method for protein complex characterization and proteome exploration. Nature Biotechnol. 17, 1030–1032 (1999).
Link, A. J. et al. Direct analysis of protein complexes using mass spectrometry. Nature Biotechnol. 17, 676–682 (1999).
Verma, R. et al. Proteasomal proteomics: identification of nucleotide-sensitive proteasome-interacting proteins by mass spectrometric analysis of affinity-purified proteasomes. Mol. Biol. Cell 11, 3425–3439 (2000).
Parks, T. D., Leuther, K. K., Howard, E. D., Johnston, S. A. & Dougherty, W. G. Release of proteins and peptides from fusion proteins using a recombinant plant virus proteinase. Anal. Biochem. 216, 413–417 (1994).
Wach, A., Brachat, A., Pohlmann, R. & Philippsen, P. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast 10, 1793–1808 (1994).
Shevchenko, A., Wilm, M., Vorm, O. & Mann, M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 68, 850–858 (1996).
Shevchenko, A. et al. Linking genome and proteome by mass spectrometry: large-scale identification of yeast proteins from two dimensional gels. Proc. Natl Acad. Sci. USA 93, 14440–14445 (1996).
Morano, K. A. & Klionsky, D. J. Differential effects of compartment deacidification on the targeting of membrane and soluble proteins to the vacuole in yeast. J. Cell. Sci. 107, 2813–2824 (1994).
Tomashek, J. J., Sonnenburg, J. L., Artimovich, J. M. & Klionsky, D. J. Resolution of subunit interactions and cytoplasmic subcomplexes of the yeast vacuolar proton-translocating ATPase. J. Biol. Chem. 271, 10397–10404 (1996).
Jackson, D. D. & Stevens, T. H. VMA12 encodes a yeast endoplasmic reticulum protein required for vacuolar H+-ATPase assembly. J. Biol. Chem. 272, 25928–25934 (1997).
Acknowledgements
We thank K. Hoffman for pointing out the F-box homologies in Ybr280 and Ymr258. We also thank D. Klionsky for antibodies against Vma1, Vma2, Vma4 and Vma8, P. Kane for monoclonal anti-Vph1 antibodies, T. Stevens for vph1Δ (KHY31) strains and polyclonal anti-Vph1 antibodies, T-M. Yi for constructing the GFP-tagging cassette, and members of the Deshaies laboratory for comments on the manuscript. This work was supported by grants from the National Institutes of Health and the W. M. Keck Foundation. J. H. S. was supported by a fellowship from the Leukemia and Lymphoma Society and by the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Seol, J., Shevchenko, A., Shevchenko, A. et al. Skp1 forms multiple protein complexes, including RAVE, a regulator of V-ATPase assembly. Nat Cell Biol 3, 384–391 (2001). https://doi.org/10.1038/35070067
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/35070067
This article is cited by
-
Identification, Genomic Organization, and Comprehensive Expression Analysis Reveals the Implication of Cicer arietinum SKP1-like Genes in Abiotic Stress
Journal of Plant Growth Regulation (2023)
-
Coordinated conformational changes in the V1 complex during V-ATPase reversible dissociation
Nature Structural & Molecular Biology (2022)
-
Innate immunity to prions: anti-prion systems turn a tsunami of prions into a slow drip
Current Genetics (2021)
-
SKP1 promotes YAP-mediated colorectal cancer stemness via suppressing RASSF1
Cancer Cell International (2020)
-
Characterization of the complex involved in regulating V-ATPase activity of the vacuolar and endosomal membrane
Journal of Bioenergetics and Biomembranes (2017)