Abstract
Natural killer (NK) cells attack many tumour cell lines, and are thought to have a critical role in anti-tumour immunity1,2,3,4,5,6,7; however, the interaction between NK cells and tumour targets is poorly understood. The stimulatory lectin-like NKG2D receptor8,9,10,11,12,13 is expressed by NK cells, activated CD8+ T cells and by activated macrophages in mice11. Several distinct cell-surface ligands that are related to class I major histocompatibility complex molecules have been identified11,12,13,14, some of which are expressed at high levels by tumour cells but not by normal cells in adults11,13,15,16. However, no direct evidence links the expression of these ‘induced self’ ligands with tumour cell rejection. Here we demonstrate that ectopic expression of the murine NKG2D ligands Rae1β or H60 in several tumour cell lines results in potent rejection of the tumour cells by syngeneic mice. Rejection is mediated by NK cells and/or CD8+ T cells. The ligand-expressing tumour cells induce potent priming of cytotoxic T cells and sensitization of NK cells in vivo. Mice that are exposed to live or irradiated tumour cells expressing Rae1 or H60 are specifically immune to subsequent challenge with tumour cells that lack NKG2D ligands, suggesting application of the ligands in the design of tumour vaccines.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Trinchieri, G. Biology of natural killer cells. Adv. Immunol. 47, 187–376 (1989).
Karre, K., Ljunggren, H. G., Piontek, G. & Kiessling, R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature 319, 675–678 (1986).
Seaman, W., Sleisenger, M., Eriksson, E. & Koo, G. Depletion of natural killer cells in mice by monoclonal antibody to NK-1.1. Reduction in host defense against malignancy without loss of cellular or humoral immunity. J. Immunol. 138, 4539–4544 (1987).
van den Broek, M. et al. Decreased tumor surveillance in perforin-deficient mice. J. Exp. Med. 184, 1781–1790 (1996).
Kaplan, D. H. et al. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc. Natl Acad. Sci. USA 95, 7556–7561 (1998).
Smyth, M. J. et al. Perforin-mediated cytotoxicity is critical for surveillance of spontaneous lymphoma. J. Exp. Med. 192, 755–760 (2000).
Kim, S., Iizuka, K., Aguila, H. L., Weissman, I. L. & Yokoyama, W. M. In vivo natural killer cell activities revealed by natural killer cell-deficient mice. Proc. Natl Acad. Sci. USA 97, 2731–2736 (2000).
Vance, R. E., Tanamachi, D. M., Hanke, T. & Raulet, D. H. Cloning of a mouse homolog of CD94 extends the family of C-type lectins on murine natural killer cells. Eur. J. Immunol. 27, 3236–3241 (1997).
Ho, E. L. et al. Murine Nkg2d and Cd94 are clustered within the natural killer complex and are expressed independently in natural killer cells. Proc. Natl Acad. Sci. USA 95, 6320–6325 (1998).
Wu, J. et al. An activating immunoreceptor complex formed by NKG2D and DAP10. Science 285, 730–732 (1999).
Diefenbach, A., Jamieson, A. M., Liu, S. D., Shastri, N. & Raulet, D. H. Ligands for the murine NKG2D receptor: expression by tumor cells and activation of NK cells and macrophages. Nature Immunol. 1, 119–126 (2000).
Bauer, S. et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science 285, 727–729 (1999).
Cerwenka, A. et al. Retinoic acid early inducible genes define a ligand family for the activating NKG2D receptor in mice. Immunity 12, 721–727 (2000).
Cosman, D. et al. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity 14, 123–133 (2001).
Nomura, M. et al. Genomic structures and characterization of Rae1 family members encoding GPI-anchored cell surface proteins and expressed predominantly in embryonic mouse brain. J. Biochem. 120, 987–995 (1996).
Groh, V. et al. Broad tumor-associated expression and recognition by tumor-derived gamma delta T cells of MICA and MICB. Proc. Natl Acad. Sci. USA 96, 6879–6884 (1999).
Gays, F. et al. The mouse tumor cell lines EL4 and RMA display mosaic expression of NK-related and certain other surface molecules and appear to have a common origin. J. Immunol. 164, 5094–5102 (2000).
van Hall, T. et al. Identification of a novel tumor-specific CTL epitope presented by RMA, EL-4, and MBL-2 lymphomas reveals their common origin. J. Immunol. 165, 869–877 (2000).
Huang, A. et al. Role of bone marrow-derived cells in presenting MHC class I-restricted tumor antigens. Science 264, 961–965 (1994).
Grufman, P. & Karre, K. Innate and adaptive immunity to tumors: IL-12 is required for optimal responses. Eur. J. Immunol. 30, 1088–1093 (2000).
Dranoff, G. et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc. Natl Acad. Sci. USA 90, 3539–3543 (1993).
Groh, V. et al. Costimulation of CD8 αβ T cells by NKG2D via engagement by MIC induced on virus-infected cells. Nature Immunol. 2, 255–260 (2001).
Glas, R. et al. Recruitment and activation of natural killer (NK) cells in vivo determined by the target cell phenotype: an adaptive component of NK cell-mediated responses. J. Exp. Med. 191, 129–138 (2000).
Pende, D. et al. Identification and molecular characterization of NKp30, a novel triggering receptor involved in natural cytotoxicity mediated by human natural killer cells. J. Exp. Med. 190, 1505–1516 (1999).
Moretta, L. et al. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu. Rev. Immunol. 19, 197–223 (2001).
Medzhitov, R. & Janeway, C. A. Jr. Innate immunity: the virtues of a nonclonal system of recognition. Cell 91, 295–298 (1997).
Hart, I. R. The selection and characterization of an invasive variant of the B16 melanoma. Am. J. Pathol. 97, 587–600 (1979).
van Elsas, A., Hurwitz, A. A. & Allison, J. P. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J. Exp. Med. 190, 355–366 (1999).
Liao, N., Bix, M., Zijlstra, M., Jaenisch, R. & Raulet, D. MHC class I deficiency: susceptibility to natural killer (NK) cells and impaired NK activity. Science 253, 199–202 (1991).
Murali-Krishna, K. et al. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity 8, 177–187 (1998).
Acknowledgements
We thank C. W. McMahon, and N. Fernandez for critical reading of the manuscript; N. Fernandez for PK136 antibody; J. Egen for help with the retroviral transfection system; H. Nolla for cell sorting; J. Hsia and C. White for technical assistance; the members of the Raulet laboratory for discussions; and J. P. Allison, M. Fasso, Y. H. Chien and B. Sha for their gift of reagents and cell lines. This work was supported by grants from the National Institute of Health (D.H.R.). A.D. is a Physician Postdoctoral Fellow of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Corresponding author
Supplementary information

Figure 1
(GIF 12.9 KB)
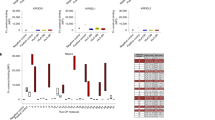
Vaccination of B6-Rag1-/- mice with ligand-expressing tumor cells does not confer specific immunity to the respective ligand-negative tumor cells. B6-Rag1-/- mice that had previously rejected ligand-transduced tumor cells (1x10 4 RMA, 1x104 B16-BL6 or 5x106 EL4 transductants) were inoculated subcutaneously with untransduced tumor cells of the same type (RMA: 1x105; B16-BL6: 1x104; EL4: 5x106). Primary exposure occurred 8-12 weeks before challenge.

Figure 2
(GIF 46.5 KB)
CTL responses primed by irradiated ligand-transduced B16-BL6 cells. Mice were vaccinated twice with 5x106 irradiated B16 transductants or PBS. Three weeks later splenocytes were restimulated with irradiated B16-Rae1b cells. a, Lysis of B16-BL6 cells. b, Target cell expression of Rae1b resulted in enhanced CTL lysis. c, d, Complement mediated-depletion of CD8 cells but not NK cells abrogates activity of effector cells from B16-Rae1b vaccinated mice. e, Elevated percentage of B16-specific IFN-g producing effector CD8+CD3+ T cells in mice primed with ligand-transduced B16 cells. Priming cells indicated at bottom. f, Expression of Rae1b by target cells enhances IFN-g response. The enhancement was completely blocked by anti-NKG2D antibody. g, h, Enhanced lysis of target cells expressing Rae1b is blocked by anti-NKG2D antibody. i, CTL priming occurs in the absence of NK cells. Mice were depleted of NK1.1+ or CD8+ cells prior to and during vaccination with tumor cell transductants. Effector cells were tested for lytic activity and IFN-g production versus B16-Rae1b target cells. j, CTL from B16-Rae1b vaccinated mice specifically recognize B16 cells and remain tolerant of syngeneic T cell blasts. Effector cells from B16-Rae1b vaccinated mice were tested for lysis of the indicated tumor cells as well as Con A blasts from syngeneic mice. Data are representative of three experiments.

Figure 3
(GIF 40.7 KB)
Induction of NK cell activation by RMA cells expressing NKG2D ligands does not require the presence of T and/or B cells, as shown in B6-Rag1-/- mice. NK cells were induced in B6-Rag1-/- mice as in Fig. 4 of the main paper with RMA transductants or RMA/S cells. a, b, Effector cells were conventional NK cells. c, d, Induction of NK cells in B6-Rag1-/- mice by ligand-transduced cells was blocked by injection of a non-depleting anti-NKG2D antiserum just prior to tumor cell inoculation. Lysis of YAC-1 tumor cells and production of IFN-g after stimulation with YAC-1 target cells were assayed. Data are representative of two experiments.

Figure 4
(GIF 11.8 KB)
B16 or EL4 cells expressing NKG2D ligands stimulate NK cell recruitment and activation in vivo. Groups of five B6 mice were injected intraperitoneally with 5x106 irradiated tumor cell transductants or PBS. Peritoneal wash cells were recovered two days later. Compared to ligand-negative cells, ligand-transduced tumor cells stimulated elevated percentages of NK (NK1.1+CD3-) cells (a, d), which exhibited enhanced capacity to lyse YAC-1 target cells (b, e), and more of which produced IFN-g when stimulated with YAC-1 target cells (c, f). Effector cells were destroyed by complement lysis with anti-NK1.1 but not anti-CD8 antibody (b, e).
Rights and permissions
About this article
Cite this article
Diefenbach, A., Jensen, E., Jamieson, A. et al. Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature 413, 165–171 (2001). https://doi.org/10.1038/35093109
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35093109
This article is cited by
-
The NK cell receptor NKp46 recognizes ecto-calreticulin on ER-stressed cells
Nature (2023)
-
Targeting myeloid-derived suppressor cells in combination with tumor cell vaccination predicts anti-tumor immunity and breast cancer dormancy: an in silico experiment
Scientific Reports (2023)
-
Roles of natural killer cells in immunity to cancer, and applications to immunotherapy
Nature Reviews Immunology (2023)
-
A nonlinear transport–diffusion model for the interactions between immune system cells and HPV-infected cells
Nonlinear Dynamics (2023)
-
Is the allee effect relevant to stochastic cancer model?
Journal of Applied Mathematics and Computing (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.