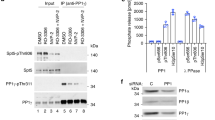
Abstract
The transcription of eukaryotic protein-coding genes involves complex regulation of RNA polymerase (Pol) II activity in response to physiological conditions and developmental cues. One element of this regulation involves phosphorylation of the carboxy-terminal domain (CTD) of the largest polymerase subunit by a transcription elongation factor, P-TEFb, which comprises the kinase CDK9 and cyclin T1 or T2 (ref. 1). Here we report that in human HeLa cells more than half of the P-TEFb is sequestered in larger complexes that also contain 7SK RNA, an abundant, small nuclear RNA (snRNA) of hitherto unknown function2,3. P-TEFb and 7SK associate in a specific and reversible manner. In contrast to the smaller P-TEFb complexes, which have a high kinase activity, the larger 7SK/P-TEFb complexes show very weak kinase activity. Inhibition of cellular transcription by chemical agents or ultraviolet irradiation trigger the complete disruption of the P-TEFb/7SK complex, and enhance CDK9 activity. The transcription-dependent interaction of P-TEFb with 7SK may therefore contribute to an important feedback loop modulating the activity of RNA Pol II.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Price, D. H. P-TEFb, a cyclin-dependent kinase controlling elongation by RNA polymerase II. Mol. Cell. Biol. 20, 2629–2634 (2000).
Zieve, G. & Penman, S. Small RNA species of the HeLa cell: metabolism and subcellular localization. Cell 8, 19–31 (1976).
Wassarman, D. A. & Steitz, J. A. Structural analyses of the 7SK ribonucleoprotein (RNP), the most abundant human small RNP of unknown function. Mol. Cell. Biol. 11, 3432–3445 (1991).
Dahmus, M. E. Reversible phosphorylation of the C-terminal domain of RNA polymerase II. J. Biol. Chem. 271, 19009–19012 (1996).
Corden, J. L. & Patturajan, M. A CTD function linking transcription to splicing. Trends Biochem. Sci. 22, 413–416 (1997).
Hirose, Y. & Manley, J. L. RNA polymerase II and the integration of nuclear events. Genes Dev. 14, 1415–1429 (2000).
Bentley, D. Coupling RNA polymerase II transcription with pre-mRNA processing. Curr. Opin. Cell. Biol. 11, 347–351 (1999).
Cassé, C., Giannoni, F., Nguyen, V. T., Dubois, M.-F. & Bensaude, O. The transcriptional inhibitors, actinomycin D and α-amanitin, activate the HIV-1 promoter and favor phosphorylation of the RNA polymerase II C-terminal domain. J. Biol. Chem. 274, 16097–16106 (1999).
Garriga, J., Mayol, X. & Grana, X. The CDC2-related kinase PITALRE is the catalytic subunit of active multimeric protein complexes. Biochem. J. 319, 293–298 (1996).
Ramanathan, Y., Reza, S. M., Young, T. M., Mathews, M. B. & Pe'ery, T. Human and rodent transcription elongation factor P-TEFb: interactions with human immunodeficiency virus type 1 Tat and carboxy-terminal domain substrate. J. Virol. 73, 5448–5458 (1999).
Devault, A. et al. MAT1 (‘ménage à trois’) a new RING finger protein subunit stabilizing cyclin H-cdk7 complexes in starfish and Xenopus CAK. EMBO J. 14, 5027–5036 (1995).
Svejstrup, J. Q., Vichi, P. & Egly, J.-M. The multiple roles of transcription/repair factor TFIIH. Trends Biochem. Sci. 21, 346–350 (1996).
Marshall, N. F. & Price, D. H. Purification of P-TEFb, a transcription factor required for the transition into productive elongation. J. Biol. Chem. 270, 12335–12338 (1995).
O'Keeffe, B., Fong, Y., Chen, D., Zhou, S. & Zhou, Q. Requirement for a kinase-specific chaperone pathway in the production of a Cdk9/cyclin T1 heterodimer responsible for P-TEFb-mediated Tat stimulation of HIV-1 transcription. J. Biol. Chem. 275, 279–287 (2000).
Yamaguchi, Y. et al. NELF, a multisubunit complex containing RD, cooperates with DSIF to repress RNA polymerase II elongation. Cell 97, 41–51 (1999).
Rockx, D. A. et al. UV-induced inhibition of transcription involves repression of transcription initiation and phosphorylation of RNA polymerase II. Proc. Natl Acad. Sci. USA 97, 10503–10508 (2000).
Egyházi, E. Initiation inhibition and reinitiation of the synthesis of heterogenous nuclear RNA in living cells. Nature 262, 319–321 (1976).
Garber, M. E. & Jones, K. A. HIV-1 Tat: coping with negative elongation factors. Curr. Opin. Immunol. 11, 460–465 (1999).
Valerie, K. A. et al. Activation of human immunodefiency virus type 1 by DNA damage in human cells. Nature 333, 78–81 (1988).
Brown, E. J. & Schreiber, S. L. A signalling pathway to translational control. Cell 86, 517–520 (1996).
Khaleghpour, K., Pyronnet, S., Gingras, A.-C. & Sonenberg, N. Translational homeostasis: eukaryotic translation initiation factor 4E control of 4E-Binding Protein 1 and p70 S6 kinase activities. Mol. Cell. Biol. 19, 4302–4310 (1999).
Ignotz, G. G., Hokari, S., DePhilip, R. M., Tsukada, K. & Lieberman, I. Lodish model and regulation of ribosomal protein synthesis by insulin-deficient chick embryo fibroblasts. Biochemistry 20, 2550–2558 (1981).
Nielsen, F. C., Ostergaard, L., Nielsen, J. & Christiansen, J. Growth-dependent translation of IGF-II mRNA by a rapamycin-sensitive pathway. Nature 377, 358–362 (1995).
Montzka Wassarman, K. & Storz, G. 6S RNA regulates E. coli RNA polymerase activity. Cell 101, 613–623 (2000).
Goodall, G. J., Wiebauer, K. & Filipowicz, W. Analysis of pre-mRNA processing in transfected plant protoplasts. Methods Enzymol. 181, 148–161 (1990).
Acknowledgements
This research was supported by grants from the Agence Nationale de Recherche sur le Sida, Association pour la Recherche sur le Cancer, the Ligue Nationale Contre le Cancer (Comité de Paris), the France-Berkeley fund and a Federation of European Biochemical Societies fellowship to A.A.M. We thank P. Ascher, M.-F. Dubois, D. H. Price and all members of the Groupe de Biologie Cellulaire de la Transcription for help and discussions, and Z. Yang and Q. Zhou for communicating their work before publication and providing the G3H cells.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nguyen, V., Kiss, T., Michels, A. et al. 7SK small nuclear RNA binds to and inhibits the activity of CDK9/cyclin T complexes. Nature 414, 322–325 (2001). https://doi.org/10.1038/35104581
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35104581
This article is cited by
-
MicroRNAs and long non-coding RNAs during transcriptional regulation and latency of HIV and HTLV
Retrovirology (2024)
-
Dengue and Zika RNA-RNA interactomes reveal pro- and anti-viral RNA in human cells
Genome Biology (2023)
-
RNF219 regulates CCR4-NOT function in mRNA translation and deadenylation
Scientific Reports (2022)
-
Poly(ADP-ribosylation) of P-TEFb by PARP1 disrupts phase separation to inhibit global transcription after DNA damage
Nature Cell Biology (2022)
-
The methyl phosphate capping enzyme Bmc1/Bin3 is a stable component of the fission yeast telomerase holoenzyme
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.