Abstract
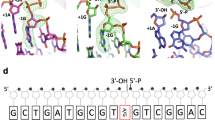
THE repair of DNA requires the removal of abasic sites, which are constantly generated in vivo both spontaneously1 and by enzymatic removal of uracil2, and of bases damaged by active oxygen species, alkylating agents and ionizing radiation3,4. The major apurinic/ apyrimidinic (AP) DNA-repair endonuclease in Escherichia coli is the multifunctional enzyme exonuclease III, which also exhibits 3′-repair diesterase, 3′→ 5′ exonuclease, 3′-phosphomonoesterase and ribonuclease activities5. We report here the 1.7 Å resolution crystal structure of exonuclease III which reveals a 2-fold symmetric, four-layered ap fold with similarities to both deoxyribo-nuclease I6 and RNase H7. In the ternary complex determined at 2.6 Å resolution, Mn2+ and dCMP bind to exonuclease III at one end of the αβ-sandwich, in a region dominated by positive electrostatic potential. Residues conserved among AP endonucleases from bacteria to man cluster within this active site and appear to participate in phosphate-bond cleavage at AP sites through a nucleophilic attack facilitated by a single bound metal ion.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Lindahl, T. Nature 362, 709–715 (1993).
Taylor, A. F. & Weiss, B. J. Bact. 151, 351–357 (1982).
Sakumi, K. & Sekiguchi, M. Mutat. Res. 236, 161–172 (1990).
Doetsch, P. W. & Cunningham, R. P. Mutat. Res. 236, 173–201 (1990).
Demple, B. & Harrison, L. A. Rev. Biochem. 63, 915–948 (1994).
Oefner, C. & Suck, D. J. molec. Biol. 192, 605–632 (1986).
Katayanagi, K. et al. Nature 347, 306–309 (1990).
Richardson, J. S. Adv. Prot. Chem. 34, 167–339 (1981).
Beese, L. S. & Steitz, T. A. EMBO J. 10, 25–33 (1991).
Lahm, A. & Suck, D. J. molec. Biol. 221, 645–667 (1991).
Weston, S. A., Lahm, A. & Suck, D. J. molec. Biol. 226, 1237–1256 (1992).
Katayanagi, K. et al. Nature 347, 306–309 (1990).
Kayana, S. et al. Eur. J. Biochem. 198, 437–440 (1991).
Katayanagi, K., Okumura, M. & Morikawa, K. Prot. Struct. Funct. Genet. 17, 337–346 (1993).
Cho, Y., Gorina, S., Jeffrey, P. D. & Pavletich, N. P. Science 265, 346–355 (1994).
Kuo, C.-F., McRee, D. E., Cunningham, R. P. & Tainer, J. A. J. molec. Biol. 229, 239–242 (1993).
Read, R. J. Acta crystallogr. A42, 140–149 (1986).
McRee, D. E. J. molec. Graphics 10, 44–46 (1993).
Brünger, A., Kuriyan, J. & Karplus, M. Science 235, 458–460 (1987).
Borgstahl, G. E. O., Rogers, P. H. & Arnone, A. J. molec. Biol. 236, 817–830 (1994).
Saporito, S. M., Smith-White, B. J. & Cunningham, R. P. J. Bact. 170, 4542–4547 (1988).
Demple, B., Herman, T. & Chen, D. S. Proc. natn. Acad. Sci. U.S.A. 88, 11450–11454 (1991).
Seki, S. et al. J. biol. Chem. 266, 20797–20802 (1991).
Robson, C. N., Milne, A. M., Pappin, D. J. C. & Hickson, I. D. Nucleic Acids Res. 19, 1087–1092 (1991).
Lipman, D. J., Altschul, S. F. & Kececioglu, J. D. Proc. natn. Acad. Sci. U.S.A. 86, 4412–4415 (1989).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mol, C., Kuo, CF., Thayer, M. et al. Structure and function of the multifunctional DNA-repair enzyme exonuclease III. Nature 374, 381–386 (1995). https://doi.org/10.1038/374381a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/374381a0
This article is cited by
-
Single 3′-exonuclease-based multifragment DNA assembly method (SENAX)
Scientific Reports (2022)
-
APE1 distinguishes DNA substrates in exonucleolytic cleavage by induced space-filling
Nature Communications (2021)
-
An Alternative Hot Start PCR Method Using a Nuclease-Deficient ExoIII from Escherichia coli
Molecular Biotechnology (2019)
-
Identification and characterization of DNA endonucleases in Plasmodium falciparum 3D7 clone
Malaria Journal (2018)
-
The mesophilic archaeon Methanosarcina acetivorans counteracts uracil in DNA with multiple enzymes: EndoQ, ExoIII, and UDG
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.