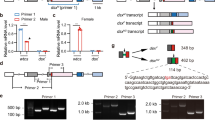
Abstract
Dosage compensation in Drosophila requires the male-specific lethal (msl) proteins (MSL) to make gene expression from the single male X chromosome equivalent to that from both female X chromosomes1,2. Expression of msl 2 is repressed post-transcrip-tionally by Sex lethal (SXL), a female-specific RNA-binding protein that regulates alternative splicing in the sex-determination hierarchy. Although msl2 RNA is alternatively spliced in males and females, this does not alter its coding potential and splicing is not required for male-specific expression of MSL2 protein. Instead, our results suggest that the association of SXL protein with multiple sites in the 5′ and 3′ untranslated regions of the mx12 transcript represses its translation in females. Thus, this well characterized alternative splicing factor regulates at least one target transcript by a distinct mechanism.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
1. Belote, J. M. & Lucchesi, J. C. Control of X chromosome transcription by the maleless gene in Drosophila. Nature 285, 573-575 (1980). 2. Baker, B. S., German, M. & Marin, I.I Dosage compensation in Drosophila. Annu. Rev. Genet. 28, 491-521 (1994). 3. Hilfiker, A., Hilfiker-Kleiner, D., Pannuti, A. & Lucchesi, J. C. mof, a putative acetyl transferase gene related to the Tip60 and MOZ human genes and to the SAS genes of yeast, is required for dosage compensation in Drosophila. EMBO J. 16, 2054-2060 (1997). 4. Kelley, R. L. et al. Expresison of Msl-2 causes assembly of dosage compensation regulators on the X chromosomes and female lethality in Drosophila. Cell 81, 867-877 (1995). 5. Cline, T. W. Autoregulatory functioning of a Drosophila gene product that establishes and maintains the sexually determined state. Genetics 107, 231-277 (1984). 6. Lucchesi, J. C. & Skripsky, T. The link between dosage compensation and sex differentiation in Drosophila melanogaster. Chromosoma 82, 217-227 (1981). 7. Zhou, S. et al. Male-specific-lethal 2, a dosage compensation gene of Drosophila that undergoes sex-specific regulation and encodes a protein with a RING finger and a metallothionein-like cluster. EMBOJ. 14, 2884-2895 (1995). 8. Bashaw, G. J. & Baker, B. S. The msl2 dosage compensation gene of Drosophila encodes a putative DNA-binding protein whose expression is sex specifically regulated by Sex-lethal. Development 121, 3245-3258 (1995). 9. Bell, L. R., Horabin, J. L, Schedl, P. & Cline, T. W. Positive autoregulation of Sex-lethal by alternative splicing maintains the female determined state in Drosophila. Cell 665, 229-239 (1991). 10. Sosnowski, B. A., Belote, J. M. & McKeown, M. Sex-specific alternative splicing of RNA from the transformer gene results from sequence-dependent splice site blockage. Cell 58, 449-459 (1989). 11. Samuels, M. E. et al. RNA binding by Sxl proteins in vitro and in vivo. Mol. Cell. Biol. 14, 4975-4990 (1994). 12. Kozak, M. Structural features in eukaryotic mRNAs that modulate the initiation of translation. /. Biol. Chem. 266, 19867-19870 (1991). 13. Geballe, A. P. & Morris, D. R. Initiation codons within 5'-leaders of mRNAs as regulators of translation. Trends Biochem. Sri. 19, 159-164 (1994). 14. Oh, S.-K., Scott, M. P. & Sarnow, P. Homeotic gene Antennapedia mRNA contains 5'-noncoding sequences that confer translational initiation by internal ribosome binding. Genes Dev. 6, 1643-1653 (1992). 15. Palmer, M. J. et al. The male specific lethal-one gene encodes a novel protein that associates with the male X chromosome in Drosophila. Genetics 134, 545-557 (1993). 16. Palmer, M. J., Richman, R., Richter, L. & Kuroda, M. I. Sex-specific regulation of the male-specific lethal-1 dosage compensation gene in Drosophila. Genes Dev. 8, 698-706 (1994). 17. Guru's, D., Lehmann, R. & Zamore, P. D. Translational regulation in development. Cell 81,171-178 (1995). 18. Caughman, S. W., Hentze, M. W., Rouault, T. A., Harford, J. B. & Klausner, R. D. The iron-responsive element is the single element responsible for iron-dependent translational regulation of ferritin biosynthesis. Evidence for function as the binding site for a translational repressor. /. Biol. Chem. 263, 19048-19052 (1988). 19. Tarun, S. Z. & Sachs, A. B. Association of the yeast poly(A) tail binding protein with translation initiation factor eIF-4G. EMBO J. 15, 7168-7177 (1996). 20. Kelley, R. L. & Kuroda, M. I. Equality for X chromosomes. Science 270, 1607-1610 (1995). 21. Pirrotta, V. in Vectors: A Survey of Molecular Cloning Vectors and Their Uses (eds Rodriguez, R. L. & Denhardt, D. T.) 437-456 (Butterworths, Boston, 1988). 22. Wang, J. & Bell, L. R. The Sex-lethal amino terminus mediates cooperative interactions in RNA binding and is essential for splicing regulation. Genes Dev. 8, 2072-2085 (1994). 23. Higuchi, R., Krummel, B. & Saiki, R. K. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 16, 7351-7367(1988). 24. Rubin, G. M. & Spradling, A. C. Genetic transformation of Drosophila with transposable element vectors. Science 218, 348-353 (1982). 25. Bone, J. R. et al. Acetylated histone H4 on the male X chromosome is associated with dosage compensation in Drosophila. Genes Dev. 8, 96-104 (1994).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kelley, R., Wang, J., Bell, L. et al. Sex lethal controls dosage compensation in Drosophila by a non-splicing mechanism. Nature 387, 195–199 (1997). https://doi.org/10.1038/387195a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/387195a0
This article is cited by
-
Absence of X-chromosome dosage compensation in the primordial germ cells of Drosophila embryos
Scientific Reports (2021)
-
Faint gray bands in Drosophila melanogaster polytene chromosomes are formed by coding sequences of housekeeping genes
Chromosoma (2020)
-
MAPCap allows high-resolution detection and differential expression analysis of transcription start sites
Nature Communications (2019)
-
Intron retention as a component of regulated gene expression programs
Human Genetics (2017)
-
Functional interplay between MSL1 and CDK7 controls RNA polymerase II Ser5 phosphorylation
Nature Structural & Molecular Biology (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.