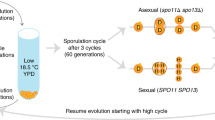
Abstract
Sex is a general feature of the life cycle of eukaryotes. It is not universal, however, as many organisms seem to lack sex entirely1. The widespread occurrence of sex is puzzling, both because meiotic recombination can disrupt co-adapted combinations of genes, and because it halves the potential rate of reproduction in organisms with strongly differentiated male and female gametes2. Most attempts to explain the maintenance of sexuality invoke differences between parents and sexual offspring. These differences may be advantageous in novel or changing environments if new gene combinations are favoured from time to time1. Sex would then serve to concentrate beneficial mutations that have arisen independently into the same line of descent. But in a stable environment sex might serve to concentrate deleterious mutations, so that they will be more effectively purged from the population by selection3. We have studied the effect of sex on mean fitness in experimental populations of the budding yeast Saccharomyces cerevisiae. Our results show that sex increases mean fitness in an environment to which the populations were well adapted, but not in an environment to which new adaptation occurred, supporting the hypothesis that the advantage of sexuality lay in the removal of deleterious mutations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Bell, G. The Masterpiece of Nature (University of California, San Francisco, (1982)).
Maynard Smith, J. The Evolution of Sex (Cambridge Univ. Press, (1978)).
Kondrashov, A. S. Deleterious mutations and the evolution of sexual reproduction. Nature 336, 435–440 (1988).
Charlesworth, D., Morgan, T. M. & Charlesworth, B. Mutation accumulation in finite outbreeding and inbreeding populations. Genet. Res. 61, 39–56 (1993).
Peck, J. R. Aruby in the rubbish: Beneficial mutations, deleterious mutations and the evolution of sex. Genetics 137, 597–606 (1994).
Malmberg, R. L. The evolution of epistasis and the advantage of recombination in populations of bacteriophage T4. Genetics 86, 607–621 (1977).
McPhee, C. P. & Robertson, A. The effect of suppressing crossing-over on the response to selection in Drosophila melanogaster. Genet. Res. 16, 1–16 (1970).
Golemis, E. A., Gyuris, J. & Brent, R. in Current Protocols in Molecular Biology (eds Ausubel, F. M. etal.) 13.1–13.14.17 (Wiley, Boston, (1994)).
Birdsell, J. & Wills, C. Significant competition advantage conferred by meiosis and syngamy in the yeast Saccharomyces cerevisiae. Proc. Natl Acad. Sci. USA 93, 908–912 (1996).
Kunz, B. A. & Haynes, R. H. in The Molecular Biology of the Yeast Saccharomyces Vol. 1(eds Strathern, J., Jones, E. W. & Broach, J.) 371–414 (Cold Spring Harbor, Plainview, (1981)).
Mortimer, R. K. & Schild, D. in The Molecular Biology of the Yeast Saccharomyces Vol. 1(eds Strathern, J., Jones, E. W. & Broach, J.) 341–370 (Cold Spring Harbor, Plainview, (1981)).
Menees, T. M. & Sandmeyer, S. B. Transposition of the yeast retroviruslike element Ty3 is dependent on the cell cycle. Mol. Cell. Biol. 14, 8229–8240 (1994).
Muller, H. L. The relation of recombination to mutational advance. Mutat. Res. 1, 2–9 (1964).
Crow, J. F. in The Evolution of Sex: an Examination of Current Ideas (eds Michod, R. E. & Levin, B. R.) 56–73 (Sinauer, Sunderland, MA, (1988)).
De Visser, J. A. G. M., Hoekstra, R. F. & Van Den Ende, H. The effect of sex and deleterious mutations on fitness in Chlamydomonas. Proc. R. Soc. Lond. B 263, 193–200 (1996).
Bilanchone, V. W. et al. Positive and negative regulatory elements control expression of the yeast retrotransposon Ty3. Genetics 134, 685–670 (1993).
Herskowitz, I. & Jensen, R. E. Putting the HO gene to work: practical uses for mating-type switching. Methods Enzymol. 194, 132–146 (1990).
Acknowledgements
We thank S. Sandmeyer for yeast strains and plasmids; H. Bussey for plasmids and the use of his micromanipulator; H. Reiswig for the use of digitizing equipment; D. Schoen for comments on the manuscript; and A. Kondrashov for helpful criticism. This research was supported by NSERC and FACR grants to G.B., NSERC and FCAR scholarships to C.Z., and grants to R. Lenski.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zeyl, C., Bell, G. The advantage of sex in evolving yeast populations. Nature 388, 465–468 (1997). https://doi.org/10.1038/41312
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/41312
This article is cited by
-
Sex alters molecular evolution in diploid experimental populations of S. cerevisiae
Nature Ecology & Evolution (2020)
-
Sexual recombination and increased mutation rate expedite evolution of Escherichia coli in varied fitness landscapes
Nature Communications (2017)
-
Fitness Effects of Thermal Stress Differ Between Outcrossing and Selfing Populations in Caenorhabditis elegans
Evolutionary Biology (2017)
-
Effect of manipulating recombination rates on response to selection in livestock breeding programs
Genetics Selection Evolution (2016)
-
Sex speeds adaptation by altering the dynamics of molecular evolution
Nature (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.