Cane toads seem to have honed their dispersal ability to devastating effect over the generations.
Abstract
Cane toads (Bufo marinus) are large anurans (weighing up to 2 kg) that were introduced to Australia 70 years ago to control insect pests in sugar-cane fields. But the result has been disastrous because the toads are toxic and highly invasive. Here we show that the annual rate of progress of the toad invasion front has increased about fivefold since the toads first arrived; we find that toads with longer legs can not only move faster and are the first to arrive in new areas, but also that those at the front have longer legs than toads in older (long-established) populations. The disaster looks set to turn into an ecological nightmare because of the negative effects invasive species can have on native ecosystems1,2; over many generations, rates of invasion will be accelerated owing to rapid adaptive change in the invader3, with continual ‘spatial selection’ at the expanding front favouring traits that increase the toads' dispersal4,5.
Similar content being viewed by others
Main
Introduced to Queensland in 1935, cane toads have since expanded their range to encompass more than a million square kilometres of tropical and subtropical Australia6. We have radio-tracked toads (for methods, see supplementary information) at the invasion front 60 km east of Darwin and confirmed the astonishing locomotor performance in these animals, which move up to 1.8 km per night during the rainy months — far further than previously studied anurans7. Does this remarkable ability result from selection for enhanced dispersal during the toads' Australian colonization history?
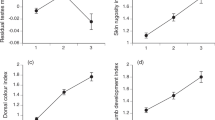
The morphological trait most often linked to locomotor ability in anurans is leg length, both among and within species8. Our trials (see supplementary information) confirm that cane toads with relatively long legs are indeed faster over a short distance (regressing time taken to cover 1 m against residual leg length: r=−0.44, n=29, P<0.02). But, more important, longer-legged toads moved further over 24 h (maximum displacement of radio-tracked toads versus relative leg length: r=0.46, n=21, P<0.04) and over three days (r=0.58, n=21, P<0.006; Fig. 1a). Longer legs therefore facilitate more rapid dispersal.
a, b, Compared with their shorter-legged conspecifics, cane toads with longer hind limbs move further over 3-day periods (r2=0.34) (a), and are in the vanguard of the invasion front (based on order of arrival at the study site; r2=0.11) (b). c, Cane toads are relatively long-legged in recent populations, and show a significant decline in relative leg length with time in older populations (r2=0.05). d, The rate at which the toad invasion has progressed through tropical Australia has increased substantially since toads were first introduced in 1935 (r2=0.92).
If the invasion process has been assisted by the evolution of improved dispersal ability among toads at the front, three consequences would be expected. First, longer-legged toads should be disproportionately common among the first wave of arrivals at any site. As the toad invasion front passed our study site, we measured relative leg lengths of all toads encountered over a 10-month period. Longer-legged toads were the first to pass through, followed by shorter-legged conspecifics (order of arrival versus relative leg length: r=−0.34, n=552, P<0.0001; Fig. 1b). Longer-legged toads therefore moved faster through the landscape.
Second, toads at the invasion front should be longer-legged than toads from older populations. As predicted, longer-term historical analysis within Queensland populations shows that relative leg length is greatest in new arrivals and then declines over a 60-year period (Fig. 1c; r=−0.23, n=139, P<0.008).
Third, the rate of progress of the toad invasion front should increase through time. As predicted, rates of frontal progress have consistently increased (Fig. 1d; time versus annual rate of spread, Pearson's r=0.96, P<0.005). Toads expanded their range by about 10 km a year during the 1940s to 1960s, but are now invading new areas at a rate of over 50 km a year. Accordingly, previous predictions about the time course of future expansion of the toads' range9 seriously underestimate their actual rates of movement.
These rapid shifts in toad morphology, locomotor speed and invasion velocity indicate that conservation biologists and managers need to consider the possibility of rapid adaptive change in invading organisms. If there is no fitness disadvantage to individual organisms at the invasion front, evolutionary forces are likely to fine-tune organismal traits in ways that facilitate more rapid expansion of the invading population10. Hence, control efforts against feral organisms should be launched as soon as possible, before the invader has had time to evolve into a more dangerous adversary.
References
Crossland, M. R. Ecography 23, 283–290 (2000).
Smith, K. G. Biol. Conserv. 123, 433–441 (2005).
Cox, G. W. Alien Species and Evolution (Island, Washington, 2004).
Simmons, A. D. & Thomas, C. D. Am. Nat. 164, 378–395 (2004).
Travis, J. M. J. & Dytham, C. Evol. Ecol. Res. 4, 1119–1129 (2002).
Lever, C. The Cane Toad. The History and Ecology of a Successful Colonist (Westbury, Otley, West Yorkshire, 2001).
Smith, M. A. & Green, D. M. Ecography 28, 110–128 (2005).
Choi, I., Shim, J. H. & Ricklefs, R. E. J. Exp. Zool. 299A, 99–102 (2003).
Freeland, W. J. & Martin, K. C. Aust. Wildl. Res. 12, 555–559 (1985).
Thomas, C. D. et al. Nature 411, 577–581 (2001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
(PDF 169 kb)
Rights and permissions
About this article
Cite this article
Phillips, B., Brown, G., Webb, J. et al. Invasion and the evolution of speed in toads. Nature 439, 803 (2006). https://doi.org/10.1038/439803a
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/439803a
This article is cited by
-
Reconstructing the biological invasion of Tuta absoluta: evidence of niche shift and its consequences for invasion risk assessment
Journal of Pest Science (2024)
-
How mutation shapes the rate of population spread in the presence of a mate-finding Allee effect
Theoretical Ecology (2023)
-
River bank burrowing is innate in native and invasive signal crayfish (Pacifastacus leniusculus) and is driven by biotic and abiotic cues
Biological Invasions (2023)
-
Body-size dependent foraging strategies in the Christmas Island flying-fox: implications for seed and pollen dispersal within a threatened island ecosystem
Movement Ecology (2022)
-
Genetic analysis of hog deer (Axis porcinus) in Victoria, Australia, and its applications to invasive species and game management
European Journal of Wildlife Research (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.