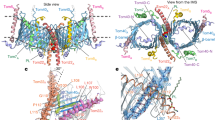
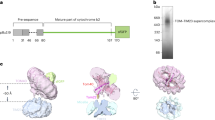
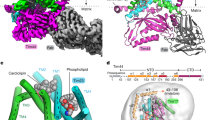
Abstract
Mitochondrial preproteins are imported by a multisubunit translocase of the outer membrane (TOM), including receptor proteins and a general import pore1,2,3,4,5. The central receptor Tom22 binds preproteins through both its cytosolic domain and its intermembrane space domain6,7,8,9,10 and is stably associated with the channel protein Tom40 (refs 11,12,13). Here we report the unexpected observation that a yeast strain can survive without Tom22, although it is strongly reduced in growth and the import of mitochondrial proteins. Tom22 is a multifunctional protein that is required for the higher-level organization of the TOM machinery. In the absence of Tom22, the translocase dissociates into core complexes, representing the basic import units, but lacks a tight control of channel gating. The single membrane anchor of Tom22 is required for a stable interaction between the core complexes, whereas its cytosolic domain serves as docking point for the peripheral receptors Tom20 and Tom70. Thus a preprotein translocase can combine receptor functions with distinct organizing roles in a multidomain protein.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Schatz,G. & Dobberstein,B. Common principles of protein translocation across membrane. Science 271, 1519–1526 (1996).
Neupert,W. Protein import into mitochondria. Annu. Rev. Biochem. 66, 863–917 (1997).
Pfanner,N., Craig,E. A. & Hönlinger,A. Mitochondrial preprotein translocase. Annu. Rev. Cell Dev. Biol. 13, 25–51 (1997).
Ryan,K. R. & Jensen,R. E. Protein translocation across mitochondrial membranes: what a long, strange trip it is. Cell 83, 517–519 (1995).
Schatz,G. Protein transport: the doors to organelles. Nature 395, 439–440 (1998).
Hönlinger,A. et al. the mitochondrial receptor complex: Mom22 is essential for cell viability and directly interacts with preproteins. Mol. Cell. Biol. 15, 3382–3389 (1995).
Bolliger,L., Junne,T., Schatz,G. & Lithgow,T. Acidic receptor domains on both sides of the outer membrane mediate translocation of precursor proteins into yeast mitochondria. EMBO J. 14, 6318–6326 (1995).
Mayer,A., Nargang,F. E., Neupert,W. & Lill,R. MOM22 is a receptor for mitochondrial targeting sequences and cooperates with MOM19. EMBO J. 14, 4205–4211 (1995).
Moczko,M. et al. The intermembrane space domain of mitochondrial Tom22 functions as a trans binding site for preproteins with N-terminal targeting sequences. Mol. Cell Biol. 17, 6574–6584 (1997).
Brix,J., Dietmeier,K. & Pfanner,N. Differential recognition of preproteins by the purified cytosolic domains of the mitochondrial import receptors Tom20, Tom22, and Tom70. J. Biol. Chem. 272, 20730–20735 (1997).
Vestweber,D., Brunner,J., Baker,A. & Schatz,G. A 42K outer-membrane protein is a component of the yeast mitochondrial protein import site. Nature 341, 205–209 (1989).
Hill,K. et al. Tom40 forms the hydrophilic channel of the mitochondrial import pore for preproteins. Nature 395, 516–521 (1998).
Dekker,P. J. T. et al. The preprotein translocase of the outer mitochondrial membrane: molecular dissection and assembly of the general import pore complex. Mol. Cell. Biol. 18, 6515–6524 (1998).
Baker,K. P., Schaniel,A., Vestweber,D. & Schatz,G. A yeast mitochondrial outer membrane protein essential for protein import and cell viability. Nature 348, 605–609 (1990).
Lithgow,T., Junne,T., Suda,K., Gratzer,S. & Schatz,G. The mitochondrial outer membrane protein Mas22p is essential for protein import and viability of yeast. Proc. Natl Acad. Sci. USA 91, 11973–11977 (1994).
Guthrie,C. & Fink,G. R. Guide to yeast genetics and molecular biology. Methods Enzymol. 194 (Academic, San Diego, California, 1991).
Nargang,F. E. et al. ‘Sheltered disruption’ of Neurospora crassa MOM22, an essential component of the mitochondrial protein import complex. EMBO J. 14, 1099–1108 (1995).
Alconada,A., Gärtner,F., Hönlinger,A., Kübrich,M. & Pfanner,N. Mitochondrial receptor complex from Neurospora crassa and Saccharomyces cerevisiae. Methods Enzymol. 260, 263–286 (1995).
Söllner,T., Rassow,J. & Pfanner,N. Analysis of mitochondrial protein import using translocaton intermediates and specific antibodies. Methods Cell Biol. 3, 345–358 (1991).
Dietmeier,K. et al. Tom5 functionally links mitochondrial preprotein receptors to the general import pore. Nature 388, 195–200 (1997).
Hönlinger,A. et al. Tom7 modulates the dynamics of the mitochondrial outer membrane translocase and plays a pathway-related role in protein import. EMBO J. 15, 2125–2137 (1996).
Alconada,A., Kübrich,M., Moczko,M., Hönlinger,A. & Pfanner,N. The mitochondrial receptor complex: the small subunit Mom8b/Isp6 supports association of receptors with the general insertion pore and transfer of preproteins. Mol. Cell. Biol. 15, 6196–6205 (1995).
Haucke,V., Horst,M., Schatz,G. & Lithgow,T. The Mas20p and Mas70p subunits of the protein import machinery interact via the tetratricopeptide repeat motif in Mas20p: evidence for a single hetero-oligomeric receptor. EMBO J. 15, 1231–1237 (1996).
Künkele, K.-P. et al. The preprotein translocation channel of the outer membrane of mitochondria. Cell 93, 1009–1019 (1998).
Kassenbrock,C. K., Cao,W. & Douglas,M. G. Genetic and biochemical characterization of ISP6, a small mitochondrial outer membrane protein associated with the protein translocation complex. EMBO J. 8, 3023–3034 (1993).
Hinnah,S., Hill,K., Wagner,R., Schlicher,T. & Soll,J. Reconstitution of a chloroplast protein import channel. EMBO J. 16, 7351–7360 (1997).
Acknowledgements
We thank M. Jacquet, T. Lithgow and T. Krimmer for materials and discussion, and H. Müller for technical assistance. This work was supported by the Sonderforschungsbereich 388, the Fonds der Chemischen Industrie (N.P.), the Sonderforschungsbereich 431 (R.W.) and long-term fellowships of the Alexander-von-Humboldt Foundation (M.T.R.) and the Human Frontier Science Program (P.J.T.D.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
van Wilpe, S., Ryan, M., Hill, K. et al. Tom22 is a multifunctional organizer of the mitochondrial preprotein translocase. Nature 401, 485–489 (1999). https://doi.org/10.1038/46802
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/46802
This article is cited by
-
Spatiotemporal stop-and-go dynamics of the mitochondrial TOM core complex correlates with channel activity
Communications Biology (2022)
-
Mapping protein interactions in the active TOM-TIM23 supercomplex
Nature Communications (2021)
-
Atomic structure of human TOM core complex
Cell Discovery (2020)
-
Mitochondrial proteins: from biogenesis to functional networks
Nature Reviews Molecular Cell Biology (2019)
-
The Principles of Protein Targeting and Transport Across Cell Membranes
The Protein Journal (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.