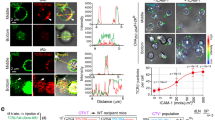
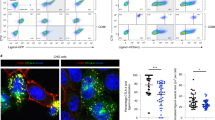
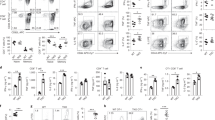
Abstract
Fas ligand (FasL) triggers apoptosis during cytotoxicity mediated by cytotoxic T lymphocytes and during immune downregulation 1 . The ability of T cells and natural killer cells to trigger apoptosis through this mechanism is controlled by the cell surface expression of FasL ( ref. 2 ). Because FasL expression is upregulated on activation 2, 3 , FasL was thought to be delivered directly to the cell surface. Here we show that newly synthesized FasL is stored in specialized secretory lysosomes in both CD4 + and CD8 + T cells and natural killer cells, and that polarized degranulation controls the delivery of FasL to the cell surface. In this way, FasL-mediated apoptosis is finely controlled by receptor-mediated target-cell recognition. The cytoplasmic tail of FasL contains signals that sort FasL to secretory lysosomes in hemopoietic cells. This pathway may provide a general mechanism for controlling the cell surface appearance of proteins involved in immune regulation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Nagata, S. & Golstein, P. The Fas death factor. Science 267, 1449–1456 (1995).
Suda, T. et al. Expression of the Fas ligand in cells of T cell lineage. J. Immunol. 154, 3806–3813 (1995).
Vignaux, F. et al. TCR/CD3 coupling to Fas-based cytotoxicity. J. Exp. Med. 181, 781–786 (1995).
Suda, T., Takahashi, T., Golstein, P. & Nagata, S. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell 75, 1169–1178 (1993).
Watanabe-Fukunaga, R. et al. The cDNA structure, expression, and chromosomal assignment of the mouse Fas antigen. J Immunol. 148, 1274–1279 (1992).
Montel, A.H., Bochan, M.R., Hobbs, J.A., Lynch, D.H. & Brahmi, Z. Fas involvement in cytotoxicity mediated by human NK cells. Cell. Immunol. 166, 236–246 (1995).
Griffith, T.S., Brunner, T., Fletcher, S.M., Green, D.R. & Ferguson, T.A. Fas ligand-induced apoptosis as a mechanism of immune priviledge [see comments]. Science 270, 1189–1192 (1995).
Peters, P.J. et al. Cytotoxic T lymphocyte granules are secretory lysosomes, containing both perforin and granzymes. J. Exp. Med. 173, 1099–1109 (1991).
Yodoi, J. et al. TCGF (IL 2)-receptor inducing factor(s). I. Regulation of IL 2 receptor on a natural killer-like cell line (YT cells). J. Immunol. 134, 1623–1630 (1985).
Kayagaki, N. et al. Metalloproteinase-mediated release of human Fas ligand. J. Exp. Med. 182, 1777–1783 (1995).
Mariani, S.M., Matiba, B., Baumler, C. & Krammer, P.H. Regulation of cell surface APO-1/Fas (CD95) ligand expression by metalloproteases. Eur. J. Immunol. 25, 2303–2307 (1995).
Isaaz, S., Baetz, K., Olsen, K., Podack, E. & Griffiths, G.M. Serial killing by cytotoxic T lymphocytes: T cell receptor triggers degranulation, re-filling of the lytic granules and secretion of lytic proteins via a non-granule pathway. Eur. J. Immunol. 25, 1071–1079 (1995).
Peters, P.J. et al. Molecules relevant for T cell-target cell interaction are present in cytolytic granules of human T lymphocytes. Eur. J. Immunol. 19, 1469–1475 (1989).
Valitutti, S., Muller, S., Dessing, M. & Lanzavecchia, A. Different responses are elicited in cytotoxic T lymphocytes by different levels of T cell receptor occupancy. J. Exp. Med. 183, 1917–1921 (1996).
Stokes, T.A. et al., Fiedler, P., Schaetzlein, C.E. & Eibel, H. & Stassi, G. et al. Technical Comments. Science 279, 2015 (1998).
Griffiths, G.M. Secretory lysosomes- a special mechanism of regulated secretion in hemopoietic in cells. Trends Cell Biol. 6, 329–332 (1996).
Griffiths, G.M. Protein sorting and secretion during CTL killing. Semin. Immunol. 9, 109–115 (1997).
Shiver, J.W. & Henkart, P.A. A noncytotoxic mast cell tumor line exhibits potent IgE-dependent cytotoxicity after transfection with the cytolysin/perforin gene. Cell 64, 1175–1181 (1991).
Hara, T., Jung, L.K., Bjorndahl, J.M. & Fu, S.M. Human T cell activation. III. Rapid induction of a phosphorylated 28 kD/32 kD disulfide-linked early activation antigen (EA 1) by 12-o- tetradecanoyl phorbol-13-acetate, mitogens, and antigens. J. Exp. Med. 164, 1988–2005 (1986).
Ziegler, S.F. et al. Molecular characterization of the early activation antigen CD69: a type II membrane glycoprotein related to a family of natural killer cell activation antigens. Eur. J. Immunol. 23, 1643–1648 (1993).
Takayama, H. & Sitkovsky, M.V. Antigen receptor-regulated exocytosis in cytotoxic T lymphocytes. J. Exp. Med. 166, 725–743 (1987).
Uellner, R. et al. Perforin is activated by a proteolytic cleavage during biosynthesis which reveals a phospholipid-binding C2 domain. EMBO J. 16, 7287–7296 (1997).
Henkart, P.A., Millard, P.J., Reynolds, C.W. & Henkart, M.P. Cytolytic activity of purified cytoplasmic granules from cytotoxic rat large granular lymphocyte tumors. J. Exp. Med. 160, 75–93 (1984).
Kiener, P.A. et al. Human monocytic cells contain high levels of intracellular Fas ligand: rapid release following cellular activation. J. Immunol. 159, 1594–1598 (1997).
Marks, M.S., Ohno, H., Kirchhausen, T. & Bonifacino, J.S. Protein sorting by tyrosine-based signals: adapting to the Ys and wherefores. Trends Cell Biol. 7, 124–128 (1997).
Henn, V. et al. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature 391, 591–594 (1998).
Acknowledgements
The authors thank J. Kaufman, G. MacPherson, J. Stinchcombe and H. Waldmann for critical reading of the manuscript and discussions. The work was supported by grants from the Wellcome Trust (040825 and 050613).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bossi, G., Griffiths, G. Degranulation plays an essential part in regulating cell surface expression of Fas ligand in T cells and natural killer cells. Nat Med 5, 90–96 (1999). https://doi.org/10.1038/4779
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/4779
This article is cited by
-
Identification of distinct cytotoxic granules as the origin of supramolecular attack particles in T lymphocytes
Nature Communications (2022)
-
The role of lysosomes in cancer development and progression
Cell & Bioscience (2020)
-
Recent trends in mucopolysaccharidosis research
Journal of Human Genetics (2019)
-
Oncolytic measles virus enhances antitumour responses of adoptive CD8+NKG2D+ cells in hepatocellular carcinoma treatment
Scientific Reports (2017)
-
Proteomic and functional analysis identifies galectin-1 as a novel regulatory component of the cytotoxic granule machinery
Cell Death & Disease (2017)