Abstract
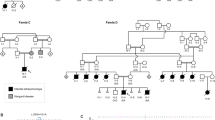
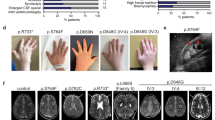
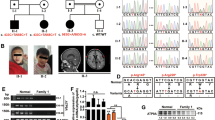
Rab GDP-dissociation inhibitors (GDI) are evolutionarily conserved proteins that play an essential role in the recycling of Rab GTPases required for vesicular transport through the secretory pathway. We have found mutations in the GDI1 gene (which encodes αGDI) in two families affected with X-linked non-specific mental retardation. One of the mutations caused a non-conservative substitution (L92P) which reduced binding and recycling of RAB3A, the second was a null mutation. Our results show that both functional and developmental alterations in the neuron may account for the severe impairment of learning abilities as a consequence of mutations in GDI1, emphasizing its critical role in development of human intellectual and learning abilities.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Lubs, H.A. et al. XLMR genes: update 1996. Am. J. Med. Genet. 64, 147–157 (1996)
Verkerk, A.J. et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell 65, 905–914 (1991)
Turner, G., Webb, T., Wake, S. & Robinson, H. Prevalence of fragile X syndrome. Am. J. Med. Genet. 64, 196–197 (1996)
Gedeon, A.K., Donnelly, A.J., Mulley, J.C., Kerr, B. & Turner, G. How many X-linked genes for non-specific mental retardation (MRX) are there? Am. J. Med. Genet. 64, 158–162 (1996)
Gecz, J., Gedeon, A.K., Sutherland, G.R. & Mulley, J.C. Identification of the gene FMR2, associated with FRAXE mental retardation. Nature Genet. 13, 105–108 (1996)
Bione, S. et al. Transcriptional organization of a 450-Kb region of the human X chromosome, in Xq28. Proc. Natl. Acad. Sci. USA 90, 10977–10981 (1993)
Wu, S.K., Zeng, K., Wilson, I.A. & Balch, W.E. Structural insights into the function of Rab GDI superfamily. Trends Biochem. Sci. 21, 472–476 (1996)
Pfeffer, S.R., Dirac-Svejstrup, A.B. & Soldati, T. Rab GDP dissociation inhibitor: putting rab GTPases in the right place. J. Biol. Chem. 270, 17057–17059 (1995)
Sasaki, T. et al. Purification and characterization from bovine brain cytosol of a protein that inhibits the dissociation of GDP from and the subsequent binding of GTP to smg p25A, a ras p21-like GTP-binding protein. J. Biol. Chem. 265, 2333–2337 (1990)
Fisher von Mollard, G., Stahl, B., Khokhlatchev, A., Suedhof, T.C. & Jahn, R. Rab3C is a synaptic vesicle protein that dissociates from synaptic vesicle after stimulation of exocytosis. J. Biol. Chem. 269, 10971–10974 (1994)
Fischer von Mollard, G., Stahl, B., Li, C., Suedhof, T.C. & Jahn, R. Rab proteins in regulated exocytosis. Trends Biochem. Sci. 19, 164–168 (1994)
Wang, Y., Okamoto, M., Schmitz, F., Hofmann, K. & Suedhof, T.C. Rim is a putative Rab3 effector in regulating synaptic-vesicle fusion. Nature 388, 593–598 (1997)
Geppert, M. et al. The role of Rab3A in neurotrasmitter release. Nature 369, 493–497 (1994)
Takai, Y., Sasaki, T., Shirataki, H. & Nakanishi, H. Rab3A small GTP-binding protein in Ca(2+)-dependent exocytosis. Genes Cells 1, 615–632 (1996)
Bean, A.J. & Scheller, R.H. Better late than never: a role for rabs late in exocytosis. Neuron 19, 751–754 (1997)
Suedhof, T.C. Function of Rab3 GDP-GTP exchange. Neuron 18, 519–522 (1997)
Sedlacek, Z., Koneki, B., Korn, B., Klauck, S.M. & Poustka, A. Evolutionary conservation and genomic organization of XA P-4, an Xq28 gene coding for a human rab GDP-dissociation inhibitor (GDI) . Mamm. Genome 5, 633–639 (1994)
Chen, E.Y. et al. Long-range sequence analysis in Xq28: thirteen known and six candidate genes in 219.4 kb of high GC DNA between the RCP/GCP and G6PD loci . Hum. Mol. Genet. 5, 659–668 (1996)
des Portes, V. et al. A gene for dominant nonspecific X-linked mental retardation is located in Xq28. Am. J. Hum. Genet. 60, 903–909 (1997)
Hamel, B.C.J. et al. A gene for non specific X-linked mental retardation (MRX41) is located in the distal segment of Xq28. Am. J. Med. Genet. 64, 131–133 (1996)
Ullrich, O. et al. Rab GDP dissociation inhibitor as a general regulator for the membrane association of rab proteins. J. Biol. Chem. 268, 18143 –18150 (1993)
Claes, S. et al. X-linked severe mental retardation and a progressive neurological disorder in a Belgian family: clinical and genetic studies. Clin. Genet. 52, 155–161 ( 1997)
Schalk, I. et al. Structure and mutational analysis of Rab GDP-dissociation inhibitor. Nature 381, 42–48 ( 1996)
Casey, P.J. & Seabra, M.C. Protein prenyltransferases. J. Biol. Chem. 271, 5289–5292 (1996)
Garrett, M.G., Zahner, J.E., Cheney, C.M. & Novick, P.J. Gdi1p encodes a GDP dissociation inhibitor that plays an essential role in the yeast secretory pathway. EMBO J. 13, 1718–1728 (1994)
Novick, P. & Zerial, M. The diversity of Rab proteins in vesicle transport. Curr. Opin. Cell Biol. 9, 496 –504 (1997)
Bachner, D., Sedlacek, Z., Korn, B., Hameister, H. & Poustka, A. Expression patterns of two human genes coding for different rab GDP-dissociation inhibitors (GDIs), extremely conserved proteins involved in cellular transport. Hum. Mol. Genet. 4, 701–708 (1995)
Banker, G.A. & Cowan, W.M. Rat hippocampal neurons in dispersed cell culture. Brain Res. 126, 397–352 (1977)
Bartlett, W.P. & Banker, G.A. An electron microscopic study of the development of axons and dendrites by hippocampal neurons in culture. II. Synaptic relationships. J. Neurosci. 4, 1954–1965 (1984)
Geppert, M., Goda, Y., Stevens, C.F. & Suedhof, T.C. The small GTP-binding protein Rab3A regulates a late step in synaptic vescicles fusion. Nature 387, 810–814 ( 1997)
Castillo, P.E. et al. Rab3A is essential for mossy fibre long-term potentiation in the hippocampus . Nature 388, 590–593 (1997)
Cremers, F.P.M., van de Pol, T.J.R., van Kerkhoff, L.P.M., Wieringa, B. & Ropers, H.H. Cloning of a gene that is rearranged in patients with choroideremia. Nature 357, 674–677 (1990).
Seabra M.C. Brown, M.S. & Goldstein, J.L. Retinal degeneration in choroideremia: deficiency of rab geranylgeranyl transferase . Science 259, 377–381 (1993)
Bione, S. et al. Identification of a novel X-linked gene responsible for Emery-Dreifuss muscular dystrophy. Nature Genet. 8, 323–327 (1994)
Spinardi, L., Mazars, R. & Theillet, C. Protocols for an improved detection of point mutations by SSCP. Nucleic Acids Res. 19, 4009 ( 1991)
Gulisano, M., Broccoli, V., Pardini, C. & Boncinelli, E. Emx1 and Emx2 show different patterns of expression during proliferation and differentiation of the developing cerebral cortex in the mouse. Eur. J. Neurosci. 8, 1037–1050 (1996)
Burgaya, F., Menegon, A., Menegoz, M., Valtorta, F. & Girault, J.A. Focal adhesion kinase in rat central nervous system . Eur. J. Neurosci. 7, 1810–1821 (1995)
Dascher, C. & Balch, W.E. Dominant inhibitory mutants of ARF1 block endoplasmic reticulum to Golgi transport and trigger disassembly of the Golgi apparatus. J. Biol. Chem. 269, 1437–1448 (1994)
Nuoffer, C., Peter, F. & Balch, W.E. Purification of His6-tagged Rab1 proteins using bacterial and insect cell expression systems. Methods Enzymol. 257, 3–8 (1995)
Fisher von Mollard, G., Suedhof, T.C. & Jahn R. Nature 349, 79–81 (1991).
Dekker, L.V., De Graan, P.N.E., Pijnappel, P., Oestreicher, A.B. & Gispen, W.H. Noradrenaline release from streptolysin O-permeated rat cortical synaptosomes: effects of calcium, phorbol esters, protein kinase inhibitors, and antibodies to the neuron-specific protein kinase C substrate B-50 (GAP-43). J. Neurochem. 56, 1146–1153 (1991)
Billuart, P. et al. Oligophrenin-1 encodes a rhoGAP protein involved in X-linked mental retardation. Nature 392, 923–926 (1998)
Acknowledgements
We thank P. Willems and A. Meindl for the DNA of patients MRX25 and MRX28, M. Zerial for the gift of the anti-αGDI antibodies, D. Dunlop for help with the computer program, E. Boncinelli for the hospitality to do in situ hybridizations; M. Gatti for skillful technical assistance, K. Zeng for preparation of the illustrations showing structural changes in αGDI and the Galliera Genetic Bank (program C23) for the patient samples. This work was funded by Telethon Italy (D.T. and F.V.) and by GM33301 and EY11606 (W.E.B.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
D'Adamo, P., Menegon, A., Lo Nigro, C. et al. Mutations in GDI1 are responsible for X-linked non-specific mental retardation. Nat Genet 19, 134–139 (1998). https://doi.org/10.1038/487
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/487
This article is cited by
-
Duplication within two regions distal to MECP2: clinical similarity with MECP2 duplication syndrome
BMC Medical Genomics (2023)
-
Astrocytes in human central nervous system diseases: a frontier for new therapies
Signal Transduction and Targeted Therapy (2023)
-
Allelic variants between mouse substrains BALB/cJ and BALB/cByJ influence mononuclear cardiomyocyte composition and cardiomyocyte nuclear ploidy
Scientific Reports (2020)
-
RAB37 interacts directly with ATG5 and promotes autophagosome formation via regulating ATG5-12-16 complex assembly
Cell Death & Differentiation (2017)
-
Impaired αGDI Function in the X-Linked Intellectual Disability: The Impact on Astroglia Vesicle Dynamics
Molecular Neurobiology (2017)