Abstract
The actin cytoskeleton seems to play two critical roles in the activation of T cells. One of these roles is T cell shape development and movement, including formation of the immunological synapse. The other is the formation of a scaffold for signaling components. This review focuses on the recent convergence of cell biology and immunology studies to explain the role of the actin cytoskeleton in creating the molecular basis for immunological synapse formation and T cell signaling.
Similar content being viewed by others
Main
Two roles of the actin cytoskeleton in T cell activation
Over 20 years ago, studies on the ligand-induced movement of immunoglobulin on the surface of B cells called attention to a special relationship between the actin cytoskeleton and antigen receptors1,2,3. Recent studies of T cells have focused our attention again on this special relationship. Actin filaments play at least two important roles in antigen recognition. The first is micrometer-scale molecular movements on the surface of the T cell4,5. This large-scale molecular rearrangement results in formation of an immunological synapse organized into distinct supramolecular activation clusters5,6. The second role of actin filaments appears to involve signaling complexes that are dependent on a scaffold of actin filaments7,8,9,10,11,12. The actin scaffold may also recruit or stabilize specialized membrane domains enriched in glycolipids and signaling molecules that are implicated in T cell activation13,14,15.
Role of actin filaments in initial adhesion
The formation of the immunological synapse is a multistep process that begins with adhesion between the T cell and antigen presenting cell (APC). Theoretically, it is possible that the T cell antigen receptor (TCR) could initiate this process by interacting with major histocompatibility complex molecule (MHC)-peptide complexes, but in practice this would require too many MHC-peptide complexes and too much time16,17. Instead, the initial adhesion is mediated by integrins such as LFA-1 or by non-integrin molecules such as CD2-CD58 or DCSIGN–ICAM-318,19,20,21. The common goal of these adhesion mechanisms is to overcome the barrier to close cell-cell contact posed by the negatively charged glycocalyx of the T cell and APC22,23. Both the T cell and APC have large glycocalyx components such as the mucin CD43, which come into conflict with each other at a ∼50–100 nm separation of cell membranes24. This distance cannot be spanned by the TCR and MHC-peptide complex, which interact at ∼15 nm25,26. One solution to this problem is to use abundant adhesion molecules—such as CD2 and CD58—to bring cells to within 15 nm of each other, by formation of thousands of transient, low affinity interactions23,27,28,29,30. T cells are sensitive to small numbers of MHC-peptide complexes on the APC31. Precise alignment of apposing membranes at ∼15 nm is probably essential for this high sensitivity. However, small adhesion molecules such as CD2 and CD58 are also prevented by the glycocalyx from interacting and require active processes to initiate adhesion32. The integrin LFA-1 and its major immunoglobulin superfamily ligand ICAM-1 can interact at ∼40 nm and can thereby initiate adhesive interactions when appropriately activated33,34. These large adhesion molecules initiate active mechanisms to promote the interaction of smaller adhesion molecules, bringing the actin cytoskeleton to center stage.
The actin cytoskeleton plays a dual role in regulation of LFA-1. The resting leukocyte has two surface domains: low flat surfaces and microvilli. Integrins such as LFA-1 are located on the low flat surface along with other large glycoproteins like CD4335 (Fig. 1). In contrast, L-selectin is located at the ends of the microvilli where they are well positioned to initiate interactions with ligands on endothelial cells36. The flat surface is supported by a cortical actin cytoskeleton that is interlaced with microtubules and intermediate filaments37. LFA-1 on resting leukocytes is maintained in a low activity state by an inhibitory interaction with the cortical actin cytoskeleton38,39,40. This inhibitory interaction may be mediated in part by talin, a large actin and integrin binding protein41. The inhibitory interaction prevents lateral movement of the integrins in the membrane that may be important for encountering ligands and clustering of integrins. In this state the lymphocyte is not well prepared for antigen recognition and requires non-antigen dependent signals to rearrange its surface topology for high sensitivity to MHC-peptide complexes.
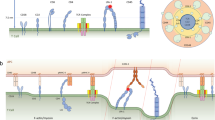
Conversion of a spherical resting T cell to a polarized migratory T cell. Actin is in green, microtubules in blue and myosin II in red. Right arrows indicate movements of cell surface structures on the lymphocytes that have just been exposed to a chemokine gradient (Δ). Chemokine receptor on naïve T cells include CCR7, which binds SLC and ELC; and CXCR4, which binds SDF-1. The microvilli are swept to the back of the cell along with CD43. Arrows on left hand diagram indicate direct of force exerted by actin polymerization on the leading edge.
Activation of lymphocytes releases the inhibitory cytoskeletal interaction, so that LFA-1 can diffuse in the plane of the membrane38. At this stage it is likely that activating signals are non-antigen-specific signals from chemokines or other environmental cues that link the preparation for antigen recognition to specific environments (for example, lymph nodes) or processes (for example, inflammation)42,43. Chemokine signaling activates a heterotrimeric G-protein–initiated signaling cascade that activates phospholipase C, phosphatidylinositol-3-kinase, protein kinase C and Rho family G-proteins leading to activation of myosin light chain kinase and local actin polymerization44. Activation of myosin II, the conventional form of myosin that forms bipolar “thick filaments”, initially increases the cortical stiffness of the leukocyte by contracting the cortical actin shell45. Further contraction by myosin II results in the collapse of the original cortical actin cytoskeleton to the side of the cell occupied by the microtubule organizing center (Fig. 1). This retraction of the resting cell cortical actin cytoskeleton creates an opening for new actin polymerization to form membrane protrusions at the leading edge of the newly polarized and motile lymphocyte. The myosin II and actin aggregate in the new trailing edge along with the microvilli and a number of proteins, including the large glycocalyx component CD43, through its interaction with the adapter protein ezrin46. The movement of CD43 to the rear edge of the cell may promote detachment of adhesion at the rear of the cells, while promoting interactions at the leading edge by relieving steric inhibition. The activation-induced polarization of the lymphocyte is functionally important. Lymphocytes are more sensitive to MHC-peptide complexes or anti-TCR antibody-induced activation at the leading edge than at the trailing edge47. Although initial polarization is stimulated by chemokines, long-term exposure to cytokines such as interleukin 2 maintains the polarized phenotype, even in the absence of external chemotactic gradients48.
A number of properties of the leading edge contribute to efficient receptor-ligand interactions. New actin polymerization is concentrated in leading protrusions and receptors in these regions are highly mobile49. Actin structures in these regions also respond rapidly to receptor engagement. For example, interaction of a mobile integrin with ligand triggers a new, adhesion-stabilizing, interaction with the actin cytoskeleton50. It has been suggested that this positive interaction may be mediated by α-actinin, an integrin and an actin binding protein distinct from talin41. Talin may also participate at this stage by stabilizing the LFA-1 clusters and the high affinity form of LFA-151,52. Cytoskeletal interactions with engaged integrins trigger expansion of close contact areas53. The integrin clusters are also transported in a directed fashion resulting in movement of the T cell over the substrate or APC54,55. Areas adjacent to active integrin clusters are forced into close contact with the apposing membrane5. These areas of forced close membrane apposition are probably the nucleation sites for interaction between smaller adhesion mechanisms and antigen receptors, and could be characterized as sensory contact domains.
The generation of sensory contact domains probably involves formation of actin-based protrusions, such as filipodia (thin spikes) or lamellipodia (flat sheets) (Fig. 1). These protrusions generate force based on actin polymerization56. The protrusive force exerted by these structures is counter-balanced by the anchoring force of the integrin interactions at the center of the contact area5. The formation of actin protrusions is controlled by small G-proteins of the Rho family including Cdc42 and RhoA57. RhoA is inactivated by the C3 ribosyltransferase. The C3 ribosyltransferase blocks LFA-1 activation and also inhibits production of interleukin 2 and sustained Ca2+ elevation in response to TCR engagement58,59,60. An efficient scheme for moving antigen receptors to within ligand binding range of the apposing membrane would be to nucleate actin assembly from the antigen receptors themselves. A link between the TCR and actin-based structures may be formed by VASP, an actin filament–coupling protein. One mechanism for linking VASP to the TCR is through the adapter protein Fyb that interacts with Fyn and VASP61,62. There are probably other mechanisms for forming this linkage as T cells from Fyn-deficient mice have normal sensitivity to antigen63. VASP is associated with protrusive structures employed in initial interactions of epithelial cells and thus may provide an appropriate linkage to actin to initiate TCR engagement with ligands64. Regardless of this, the first critical role for the actin cytoskeleton appears to be collaboration with chemokine receptor–mediated signals and adhesion molecules to initiate physiological TCR engagement.
Formation of the synapse
When agonist MHC-peptide complexes are present on an antigen-presenting surface with integrin ligands, the coordination of adhesion, the actin cytoskeleton and antigen receptors takes on a different character. The T cell stops migrating65,66 and generates a central area of integrin engagement surrounded by a close-contact region that includes the bulk of engaged TCR5 (Fig. 2a). Over a period of minutes the engaged TCR are transported to the center of the contact area and the engaged integrins are forced into a surrounding ring (Fig. 2b)5,6. This pattern can be stable for several hours and sustained signaling on this timescale is required for full T cell activation (Fig. 2c)67. Tracking of TCR on the T cell surface reveals an initial bipolar distribution. Small clusters form in the nascent synapse in response to MHC-peptide complex interaction, with one large cluster at the opposite pole of the cell. This paradoxical clustering at the cell pole away from the APC is probably due to myosin II activation and actin filament contraction at the original trailing edge (see Web movies 1–4; http://dx.doi.org/10.1038/20000000). Over a period of several minutes the small TCR clusters rearrange to form a large central cluster within the immunological synapse and additional TCRs move from the distal pole into the central cluster of the synapse. The transport of receptors to the immunological synapse was discovered by Wülfing and Davis and requires myosin II4. Therefore in the motile T cell, myosin II is involved in generating a flow of actin towards the rear of the cell, which is reversed on synapse formation. This reversal in the polarity of actin movement requires engagement of the TCR along with either LFA-1 or CD284. The formation of the immunological synapse organization is sensitive to the density and quality of MHC-peptide complexes5,6. Therefore, the process of forming the immunological synapse involves the entire T cell surface and the actin and myosin cytoskeletal systems to translate the short-lived interaction of TCR and MHC-peptide complexes into a stable supramolecular structure of cell-sized proportions.
(a–b) Schematic model for immunological synapse formation linked to details of actin dynamics. MHC-peptide complexes are green and ICAM-1 is red. The boxes link to insets with detailed description of molecules linking TCR and actin at different stages of synapse formation. (a) Dendritic nucleation model of actin polymerization triggered by activation of the Arp2/3 complex in the presence of actin (clear parallelogram, ATP bound; purple parallelogram, ADP bound), capping protein (red square) and cofilin (blue triangles). Initially ATP-actin is added to the barbed end of the filament, but is then hydrolyzed to ADP + inorganic phosphate. Once this occurs the Arp2/3 complex dissociates. Cofilin then promotes the dissociation of the inorganic phosphate leading to filament fragmentation. Cofilin phosphorylation by LIM kinase inhibits actin binding and increases filament half-life. (b) The role of bipolar myosin II filaments (red) in contraction of the actin network to form the central cluster of engaged TCR. The formation of many long capped actin filaments is likely to require inhibition of cofilin. One potential mechanism is the ability of the Rac-activated form of the p21-activated kinase to active LIM kinase, which phosphorylates cofilin and inhibits its function. These more stable filaments may then be linked into the TCR associated actin by actin filament cross-linking proteins like α-actinin or L-plastin. Contraction of the actin gel by activated myosin II results in formation of the central TCR cluster. (c) The contracted dendritic actin network serves as a scaffold for protein kinese C-θ and other molecules involved in the sustained signaling process. It is presumed that specific adapters or scaffolding proteins are required for this process.
Molecular links between the TCR and actin cytoskeleton
The TCR is a multi-subunit transmembrane glycoprotein composed of the antigen binding α and β subunits, and the invariant CD3 complex and ζ-chains. The cytoplasmic domains of the CD3 and ζ polypeptides contain immune-receptor tyrosine activation motifs (ITAMs) that become phosphorylated by src family tyrosine kinases such as Lck upon TCR engagement. Each ζ subunit has three ITAMs and each ITAM has two tyrosines. The ITAMs are necessary for TCR-induced actin polymerization68. ITAM phosphorylation patterns in the ζ polypeptide are ordered such that phosphorylation of both tyrosines in an ITAM is only observed with agonist MHC-peptide complexes69. ITAMs with both tyrosines phosphorylated are required to recruit the non-receptor tyrosine kinase ZAP-70 through its paired SH2 domains70. ZAP-70 activation may be inhibited by the phosphatases SHP-1 and CD45 and degradation targeting factors c-Cbl and Cbl-b71,72,73,74,75,76. The large transmembrane tyrosine phosphatase CD45 also has a positive role in activation of Lck. These positive and negative regulators contribute to setting thresholds for T cell activation. ZAP-70 phosphorylates sites that lead to the recruitment of adapter proteins SLP-76 and LAT77,78,79,80. With respect to the actin cytoskeleton, SLP-76 is particularly unusual because it recruits Vav and Nck11. Nck recruits WASp (a deficiency of which causes Wiscott-Aldrich Syndrome). Vav activates Rho-family G-proteins Cdc42 and Rac. In its inactive state, WASp assumes a closed conformation that cannot interact with the key actin regulator Arp2/3, a complex containing actin related proteins 2 and 3 plus five other polypeptides81. Activated Cdc42 interacts with WASp to induce an open conformation that recruits and activates the Arp2/3 complex. Inositol phospholipids, in part generated by action of phosphatidyl inositol 3-kinase linked to the TCR recruited adapter molecule LAT, also appear to contribute to activation of WASp through interaction with the pleckstrin homology domain of WASp80,82. WASp also associates with a proline-rich adapter protein, WIP, which has actin and profilin binding sites and augments actin polymerization83. Activated forms of Rho family G-proteins orchestrate the formation of complex actin-based structures by activating multiple effector molecules57. Activated Cdc42 generates filipodia, fine actin containing projections, and Rac generates lamellipodia, flat areas of active actin polymerization at membranes that can appear as “ruffles” at the outer edge of the immunological synapse (see Web movies 5–7; http://dx.doi.org/10.1038/20000000). In T lymphocytes these signaling processes, as assessed by tyrosine phosphorylation and Ca2+ mobilization, are maximal during the few minutes following initial interaction with the APC when the immunological synapse is forming.
Key molecular components recruited to the activated TCR are sufficient to induce dramatic actin polymerization. Recruitment of Nck and N-WASp to Vaccinia virus particles in the cytoplasm of infected cells stimulates formation of actin “rockets” that propel particles through the cytoplasm in a process dependent only on actin polymerization84. The movement of Vaccinia particles in the cytoplasm is similar to the movement of Listeria monocytogenes in which the ActA protein on the bacterial surface directly recruits Arp2/3 to induce formation of actin rockets85,86. Listeria-based motility has now been reconstituted in a system with four purified proteins: actin, Arp2/3, capping protein and cofilin87. This study was a milestone, providing formal proof that actin polymerization generates motile force. It is thought that the mechanism of actin polymerization involves the ability of activated Arp2/3 complexes to bind the sides of existing actin filaments and to nucleate new barbed filament ends (the fast growing end) while capping the pointed end88. This “dendritic nucleation” process results in a rapidly expanding network of polymerized actin that exerts force on the particle89. The ability of WASp to stimulate this process has been observed directly in vitro82.
Vaccinia utilizes a tyrosine phosphorylated transmembrane protein to recruit Nck and N-WASp and is thus more analogous than Listeria to the antigen receptor systems. In T cells, mutations in SLP-76 or Nck result in impaired TCR-stimulated actin polymerization11. Mice deficient in Vav1 or WASp show specific defects in T cell activation and actin polymerization in response to antibody cross-linking9,10,90. Human patients deficient in WASp exhibit defects in T cell activation and actin-based structures91. These defects were observed despite the presence of two other Vav isoforms and at least one additional WASp isoform. The initial TCR clusters that form at the periphery of the nascent immunological synapse are likely to be sites of extensive dendritic nucleation growth of actin networks (Fig. 2a, inset). The immunological synapse model predicts that synapse formation will be at least partially defective in Vav- and WASp-deficient mice.
Models for sensory contact and cluster assembly
The close sensory contacts adjacent to sites of integrin engagement are probably generated by actin polymerization nucleated at the membrane by the Arp2/3 complex. Regions of the T cell surface rich in active protrusions are most sensitive to MHC-peptide complexes on APCs or to anti-CD3 on beads47,65. Therefore the protrusions should be rich in TCR. The TCR has relatively low lateral mobility on the surface of T cells with only 20% of proteins showing free diffusion5. It is likely therefore that the TCR has a basal association with the cytoskeleton that may be mediated by proteins such as Fyn, Fyb and VASP as described above. The TCR would then be forced toward the surface of the APC to make for efficient interaction with the APC surface MHC-peptide complexes.
Following antigen receptor triggering, it is clear that the TCR recruits Nck, WASp and Vav in a manner that is likely to activate the Arp2/3 complex and trigger rapid actin polymerization (Fig. 2a, inset). This polymerization may drive the MHC–peptide-engaged TCR clusters further against the APC surface to expand the area of close contact.
The mechanism by which the MHC-peptide–engaged TCR are then transported to the center of the contact to form the central TCR cluster is not known. A likely mechanism would involve the activation of myosin II. Myosin II can be activated by the interaction of Ca2+-calmodulin with myosin light chain kinase, which phosphorylates the regulatory light chain of myosin92. The bipolar myosin filaments then contract the actin filaments into a dense gel bringing along associated surface proteins (Fig. 2b, inset). This mechanism is thought to account for the flow of actin towards the rear end of cells undergoing amoeboid motion93,94,95. Stabilization of the actin filaments to provide a substrate for the myosin II based motility may be promoted by activation of the p21 activated kinase (PAK) at the TCR through the action of Vav and SLP-7611. Activated PAK in turn activates LIM kinase, which phosphorylates an inhibitory residue on cofilin96. Cofilin participates in the disassembly of actin filaments. Inhibition of cofilin should result in a longer half-life of actin filaments that could then be contracted by activated myosin II. An alternative mechanism for engaged TCR movement to the center of the synapse would be that actin polymerization itself would drive the complexes inward. Either of these hypotheses can account for the observation that the number of MHC-peptide complexes in the central compartment of the immunological synapse is related to the degree of complete ITAM phosphorylation and ZAP-70 recruitment. This is because both models are sensitive to the rate of actin filament formation stimulated at the TCR in response to ZAP-70 recruitment5,69.
How is the integrin ring maintained?
An intriguing aspect of the immunological synapse is the interdependence and coordination of adhesion and signaling complex formation. The engaged TCR and associated actin filaments move into the center of the synapse, but LFA-1—which is also functionally associated with actin—remains in a discrete peripheral ring of adhesion. This occurs even when the LFA-1 ligand, ICAM-1, is freely mobile—so anchorage to the substrate by ICAM-1 cannot explain failure of LFA-1 to also cluster in the middle. One possible mechanism is the association of LFA-1 with talin (a large cytoskeletal protein with multiple attachment sites to integrins), the cytoskeletal protein vinculin and actin filaments. Talin is strongly accumulated in areas of LFA-1 engagement within the immunological synapse6. Talin has been implicated in lateral immobilization of integrin complexes41 and may play an important role in protecting integrin-mediated contact from being swept to the center of the synapse. The adhesion molecule CD2 also appears to establish and maintain a position outside the center of the synapse, but largely inside the integrin ring5. This localization of CD2 may be stabilized by CD2AP, an adapter protein implicated in CD2 movement and formation of stable cell-cell junctions in the kidney97,98.
Modes of T cell activation
The formation of a stable synapse in the inductive phase of an immune response may serve a number of functions. For example, a migrating T cell must stop moving when it encounters antigen. The synapse would allow cytokine secretion by the T cell to be focused on an APC or target cell99. The synapse provides a structural “hardware” solution to integrating signals from the environment in the form of supramolecular compartments. It appears that these compartments are stable over several hours, and that a threshold number of MHC-peptide complexes (∼50) must be engaged in parallel to generate the structure. Therefore, this massively parallel triggering process is well geared to produce a high fidelity deterministic signal based on MHC-peptide number and quality, and the adhesion molecules on the APC (Fig. 3a). The formation of these supramolecular clusters may carefully measure MHC-peptide number and kinetics of interaction with the TCR, and thus set a rigorous threshold for full T cell activation.
(a) The parallel mode of TCR triggering results from the incorporation of triggered TCR into the central cluster. The star symbolizes signaling. Data on TCR down regulation suggest that multiple TCR are occupied at different times by each MHC-peptide complex. However, many TCR are triggered in parallel through incorporation into a higher order structure. The entire TCR cluster is degraded en bloc when the T cell and APC separate119. (b) The serial mode of TCR triggering results from the rapid dispersal of triggered TCR without incorporation into a higher order structure. The MHC-peptide complexes can be used to engage many TCR in a serial fashion.
A distinct mode of T cell signaling had been proposed earlier based on serial engagement100. In this process the TCR are triggered for signaling and then eliminated at the single receptor level (Fig. 3b). Molecular segregation in contact areas provides a coreceptor-independent mechanism for signaling initiated by single TCR engagement events101,102,103. However, serial triggering has not been directly observed in cell contacts and is indirectly studied through TCR down-regulation, a process equally compatible with serial triggering or resolution of an immunological synapse. Support for serial triggering is also provided by studies in which T cells move while signaling, even from APC to APC7,104. Although significant movement appears to rule out formation of a stable immunological synapse in these examples, the molecular organization of these mobile synapses have not been investigated. Direct observation of serial triggering would require following the natural history of single TCR molecules, which may soon be possible given current rate of progress in imaging technology105,106.
It is thought that serial-engagement requires actin polymerization to set up appropriate contact areas and signaling scaffolds (but that this is not necessary for large-scale receptor movement)7,107. This mode of signaling can achieve high sensitivity because each MHC-peptide complex can trigger many TCRs108. Such a system may receive a significant number of incorrect cell activation events due to the problem of separating the baseline of spontaneous signals from true engagement events. In primary activation of mature T cells, the cost of incorrect activation could be very high (for example, autoimmunity) so the added fidelity through formation of the synapse may be an evolutionary response to this cost. On the other hand, in developing T cells, the cost of incorrect activation in positive and negative selection may be less problematic. Incorrect activation in positive selection results in production of a mature T cell that poorly recognizes self-MHC and therefore is not likely to be activated in the periphery by any self-MHC–peptide complex. These cells would probably die off due to lack of survival signals in the periphery109. Incorrect activation during negative selection results in loss of a cell that would have been a useful mature T cell. Small numbers of such errors may not be serious because they do not generate destructive cells.
Therefore, some T cell development and possibly also mature T cell survival signals may be received in a serial, rather than parallel, manner. Serial engagement predicts that the signaling process in immature T cells is tuned to discriminate between the noise of spontaneous receptor firing, and very small numbers of isolated engagement events. This is a “software integration” process that relies on determining the rate and duration of single TCR engagement events as opposed to the “hardware integration” process of the immunological synapse, where a stable structure is formed to sustain parallel TCR signaling events. It has been proposed that CD4 or CD8 lineage decisions may be controlled by the duration of signaling in immature T cells110, and that the role of CD4 in promoting synapse formation5 may provide the long term (∼10 h) signaling required for an immature T cell expressing both CD4 and CD8 to develop into a CD4+ mature T cell. Therefore, it will be very important to determine the degree to which different types and developmental stages of T cells rely on these alternative signaling mechanisms, parallel or serial, for responding to MHC-peptide complexes in vivo.
The molecular mechanisms that tie mature T cell activation to synapse formation are not clear. It is likely that there will be differences in signaling pathways between immature and mature T cells. Specific synapse-dependent signaling molecules appear in key aspects of mature T cell activation. For example, protein kinase C-θ (PKC-θ) is required for activation of NF-κB in mature T cells, but not in immature T cells111. Although it is possible that this developmental difference in the requirement for PKC-θ is due to some redundancy in immature T cells, it is equally possible that this difference reflects a real difference in signaling mechanisms. PKC-θ is unique among PKC forms in its localization to the central supramolecular activation cluster within the immunological synapse112.
Role of actin filaments in signaling
Disruption of actin filaments blocks T cell activation immediately7,8,113. One hypothesis for this effect is that actin filaments establish a scaffold for assembly of signaling complexes. Adapter proteins would be involved in linking specific signaling pathways to actin as has been demonstrated in other systems114,115. One candidate for recruitment through the actin scaffold is PKC-θ, the kinase recruited to the center of the mature synapse (Fig. 2c, inset). PKC-θ activity is dependent on Vav, but its recruitment to the TCR cluster appears to be indirect and is dependent on the ability of Vav to induce actin polymerization12. The actin scaffold may also recruit or stabilize glycolipid-rich rafts that concentrated in the vicinity of the engaged TCR14. The impact of the actin scaffold may be to sustain signaling complexes by protecting components from degraadation. For example, CD28 co-engagement with the TCR is required for effective raft recruitment in primary stimulation of mature T cells. Cbl-b is a ring finger protein that functions in suppression of signaling by acting as a ubiquitin ligase to target neighboring proteins for degradation by the proteosome73,74. The Cbl-b−/− mouse has a hyper-responsive T cell phenotype that can be alleviated by crossing to the CD28−/− mouse. One could speculate that the role of CD28 in organizing the cytoskeleton in the immunological synapse may be, in part, to protect certain signaling molecules from proteolysis by linking them to the actin scaffold, and thus prolonging signals from engaged TCR. The contribution of the actin scaffold to sustained signaling is probably important for full T cell activation which can require up to 20 h of sustained signaling67. The kinetics of APC clusters with T cells in vivo are also consistent with an important role of sustained signaling in vivo116,117.
Conclusions
Since the classic experiments of Mechnikoff on the attack of ameobocytes on foreign bodies118, immunologists have appreciated the importance of cell motility and cytoarchitecture to the immune response. Our current understanding of the actin cytoskeleton is based on information gathered from a large number of model systems as diverse as biochemistry of soil amoeba, genetics of yeast, and microscopy of fish keratocytes. Advances in protein chemistry, mammalian genetics and imaging technologies have allowed the applications of lessons from these model systems to the function of specific cytoskeletal proteins in T lymphocytes paired with antigen presenting systems in vitro, or in the context of the entire organism. The ability to formulate and test hypotheses based on actin mechanisms shared by diverse organisms should lead to further identification and discovery of unique aspects of the lymphocyte cytoskeletal–signaling system that may provide tomorrow's immunological therapies.
References
Unanue, E. R., Perkins, W. D. & Karnovsky, M. J. Ligand-induced movement of lymphocyte membrane macromolecules. I. Analysis by immunofluorescence and ultrastructural radioautography. J. Exp. Med. 136, 885–906 (1972).
Bourguignon, L. Y. W. & Singer, S. J. Transmembrane interactions and the mechanism of capping of surface receptors by their specific ligands. Proc. Natl Acad. Sci. USA 74, 5031–5035 (1977).
Braun, J., Fujiwara, K., Pollard, T. D. & Unanue, E. R. Two distinct mechanisms for redistribution of lymphocyte surface macromolecules. I. Relationship to cytoplasmic myosin. J. Cell Biol. 79, 409–418 (1978).
Wülfing, C. & Davis, M. M. A Receptor/Cytoskeletal movement triggered by costimulation during T cell activation. Science 282, 2266–2269 (1998).
Grakoui, A. et al. The immunological synapse: A molecular machine controlling T cell activation. Science 285, 221–227 (1999).
Monks, C. R., Freiberg, B. A., Kupfer, H., Sciaky, N. & Kupfer, A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature 395, 82–86 (1998).
Valitutti, S., Dessing, M., Aktories, K., Gallati, H. & Lanzavecchia, A. Sustained signalling leading to T cell activation results from prolonged T cell receptor occupancy. Role of T cell actin cytoskeleton. J. Exp. Med. 181, 577–584 (1995).
Delon, J., Bercovici, N., Liblau, R. & Trautmann, A. Imaging antigen recognition by naive CD4+ T cells: compulsory cytoskeletal alterations for the triggering of an intracellular calcium response. Eur. J. Immunol. 28, 716–729 (1998).
Fischer, K. D. et al. Vav is a regulator of cytoskeletal reorganization mediated by the T- cell receptor. Curr. Biol. 8, 554–562 (1998).
Holsinger, L. J. et al. Defects in actin-cap formation in Vav-deficient mice implicate an actin requirement for lymphocyte signal transduction. Curr. Biol. 8, 563–572 (1998).
Bubeck Wardenburg, J. et al. Regulation of PAK activation and the T cell cytoskeleton by the linker protein SLP-76. Immunity 9, 607–616 (1998).
Villalba, M. et al. A novel functional interaction between Vav and PKCθ is required for TCR-induced T cell activation. Immunity 12, 151–160 (2000).
Xavier, R., Brennan, T., Li, Q., McCormack, C. & Seed, B. Membrane compartmentation is required for efficient T cell activation. Immunity 8, 723–732 (1998).
Viola, A., Schroeder, S., Sakakibara, Y. & Lanzavecchia, A. T lymphocyte costimulation mediated by reorganization of membrane microdomains. Science 283, 680–682 (1999).
Harder, T. & Simons, K. Clusters of glycolipid and glycosylphosphatidylinositol-anchored proteins in lymphoid cells: accumulation of actin regulated by local tyrosine phosphorylation. Eur. J. Immunol. 29, 556–562 (1999).
Wülfing, C., Sjaastad, M. D. & Davis, M. M. Visualizing the dynamics of T cell activation: intracellular adhesion molecule 1 migrates rapidly to the T cell/B cell interface and acts to sustain calcium levels. Proc. Natl Acad. Sci. USA 95, 6302–6307 (1998).
Dustin, M. L. et al. T cell receptor mediated adhesion of T cell hybridomas to planar bilayers containing purified MHC class II/peptide complexes and receptor shedding during detachment. J. Immunol. 157, 2014–2021 (1996).
Martz, E. LFA-1 and other accessory molecules functioning in adhesions of T and B lymphocytes. Hum. Immunol. 18, 3–37 (1987).
Springer, T. A. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu. Rev. Physiol. 57, 827–872 (1995).
Wang, J. H. et al. Structure of a heterophilic adhesion complex between the human CD2 and CD58 (LFA-3) counterreceptors. Cell 97, 791–803 (1999).
Geijtenbeek, T. B. et al. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell 100, 575–585 (2000).
Springer, T. A. Adhesion receptors of the immune system. Nature 346, 425–433 (1990).
Shaw, A. S. & Dustin, M. L. Making the T cell receptor go the distance: a topological view of T cell activation. Immunity 6, 361–369 (1997).
Cyster, J. G., Shotton, D. M. & Williams, A. F. The dimensions of the T lymphocyte glycoprotein leukosialin and identification of linear protein epitopes that can be modified by glycosylation. EMBO J. 10, 893–902 (1991).
Garcia, K. C. et al. An Alpha-Beta T Cell Receptor structure at 2.5 Å and its orientation in the TCR-MHC complex. Science 274, 209–219 (1996).
Garboczi, D. N. et al. Structure of the complex between human T-cell receptor, viral peptide and HLA-A2. Nature 384, 134–141 (1996).
Dustin, M. L., Ferguson, L. M., Chan, P. Y., Springer, T. A. & Golan, D. E. Visualization of CD2 interaction with LFA-3 and determination of the two-dimensional dissociation constant for adhesion receptors in a contact area. J. Cell Biol. 132, 465–474 (1996).
Davis, S. J. & van der Merwe, P. A. The structure and ligand interactions of CD2: implications for T-cell function. Immunol. Today 17, 177–187 (1996).
Dustin, M. L. Adhesive bond dynamics in contacts between T lymphocytes and glass supported planar bilayers reconstituted with the immunoglobulin related adhesion molecule CD58. J. Biol. Chem. 272, 15782–15788 (1997).
Wild, M. K. et al. Dependence of T cell antigen recognition on the dimensions of an accessory receptor-ligand complex. J. Exp. Med. 190, 31–41 (1999).
Peterson, D. A., DiPaolo, R. J., Kanagawa, O. & Unanue, E. R. Negative selection of immature thymocytes by a few peptide-MHC complexes: differential sensitivity of immature and mature T cells. J. Immunol. 162, 3117–3120 (1999).
Chan, P. Y. & Springer, T. A. Effect of lengthening lymphocyte function-associate 3 on adhesion to CD2. Mol. Biol. Cell 3, 157–166 (1992).
Staunton, D. E., Dustin, M. L., Erickson, H. P. & Springer, T. A. The arrangement of the immunoglobulin-like domains of ICAM-1 and the binding sites for LFA-1 and rhinovirus. Cell 61, 243–254 (1990).
Nermut, M. V., Green, N. M., Eason, P., Yamada, S. S. & Yamada, K. M. Electron microscopy and structural model of human fibronectin receptor. EMBO J. 7, 4093–4099 (1988).
Sanchez-Madrid, F. & del Pozo, M. A. Leukocyte polarization in cell migration and immune interactions. EMBO J. 18, 501–511 (1999).
von Andrian, U. H., Hasslen, S. R., Nelson, R. D., Erlandsen, S. L. & Butcher, E. C. A central role for microvillous receptor presentation in leukocyte adhesion under flow. Cell 82, 989–999 (1995).
Albrecht, D. L., Mills, J. W. & Noelle, R. J. Membrane Ig-cytoskeletal interactions. III. Receptor cross-linking results in the formation of extensive filamentous arrays of vimentin. J. Immunol. 144, 3251–3256 (1990).
Kucik, D. F., Dustin, M. L., Miller, J. M. & Brown, E. J. Adhesion activating phorbol ester increases the mobility of leukocyte integrin LFA-1 in cultured lymphocytes. J. Clin. Invest. 97, 2139–2144 (1996).
Lub, M., van Kooyk, Y., van Vliet, S. J. & Figdor, C. G. Dual role of the actin cytoskeleton in regulating cell adhesion mediated by the integrin lymphocyte function associated antigen-1. Mol. Biol. Cell 8, 341–351 (1997).
Stewart, M. P., McDowall, A. & Hogg, N. LFA-1-mediated adhesion is regulated by cytoskeletal restraint and by a Ca2+-dependent protease, calpain. J. Cell Biol. 140, 699–707 (1998).
Sampath, R., Gallagher, P. J. & Pavalko, F. M. Cytoskeletal interactions with the leukocyte integrin β2 cytoplasmic tail. Activation-dependent regulation of associations with talin and α-actinin. J. Biol. Chem. 273, 33588–33594 (1998).
Campbell, J. J., Qin, S., Bacon, K. B., Mackay, C. R. & Butcher, E. C. Biology of chemokine and classical chemoattractants receptors: differential requirements for adhesion-triggering versus chemotactic responses in lymphoid cells. J. Cell Biol. 134, 255–266 (1996).
Hwang, S. T. et al. GlyCAM-1, a physiologic ligand for L-selectin, activates β 2 integrins on naive peripheral lymphocytes. J. Exp. Med. 184, 1343–1348 (1996).
Servant, G. et al. Polarization of chemoattractant receptor signaling during neutrophil chemotaxis . Science 287, 1037–1040 (2000).
Elson, E. L. et al. Activation of mechanical responses in leukocytes. Biorheology 27, 849–858 (1990).
Serrador, J. M. et al. CD43 interacts with moesin and ezrin and regulates its redistribution to the uropods of T lymphocytes at the cell-cell contacts. Blood 91, 4632–4644 (1998).
Wei, X., Tromberg, B. J. & Cahalan, M. D. Mapping the sensitivity of T cells with an optical trap: polarity and minimal number of receptors for Ca(2+) signaling. Proc. Natl Acad. Sci. USA 96, 8471–8476 (1999).
Wilkinson, P. C. The locomotor capacity of human lymphocytes and its enhancement by cell growth. Immunology 57, 281–289 (1986).
Kucik, D. F., Kuo, S. C., Elson, E. L. & Sheetz, M. P. Preferential attachment of membrane glyocoproteins to the cytoskeleton at the leading edge of Lamella. J. Cell Biol. 114, 1029–1036 (1991).
Felsenfeld, D. P., Choquet, D. & Sheetz, M. P. Lingand binding regulates the directed movement of β1 integrins on fibroblasts. Nature 383, 438–440 (1996).
Sedwick, C. E. et al. TCR, LFA-1, and CD28 play unique and complementary roles in signaling T cell cytoskeletal reorganization. J. Immunol. 162, 1367–1375 (1999).
Calderwood, D. A. et al. The Talin head domain binds to integrin beta subunit cytoplasmic tails and regulates integrin activation. J. Biol. Chem. 274, 28071–28074 (1999).
Stewart, M. P., Cabanas, C. & Hogg, N. T cell adhesion to intercellular adhesion molecule-1 (ICAM-1) is controlled by cell spreading and the activation of integrin LFA-1. J. Immunol. 156, 1810–1817 (1996).
Chang, T. W., Celis, E., Eisen, H. N. & Solomon, F. Crawling movements of lymphocytes on and beneath fibroblasts in culture. Proc. Natl Acad. Sci. USA 76, 2917–2921 (1979).
Lauffenburger, D. A. & Horwitz, A. F. Cell migration: a physically integrated molecular process. Cell 84, 359–369 (1996).
Borisy, G. G. & Svitkina, T. M. Actin machinery: pushing the envelope. Curr. Opin. Cell Biol. 12, 104–112 (2000).
Nobes, C. D. & Hall, A. Rho, Rac, and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filipodia. Cell 81, 53–62 (1995).
Tominaga, T. et al. Inhibition of PMA-induced, LFA-1-dependent lymphocyte aggregation by ADP ribosylation of the small molecular weight GTP binding protein, rho. J. Cell Biol. 120, 1529–1537 (1993).
Laudanna, C., Campbell, J. J. & Butcher, E. C. Role of Rho in chemoattractant-activated leukocyte adhesion through integrins. Science 271, 981–983 (1996).
Angkachatchai, V. & Finkel, T. H. ADP-ribosylation of rho by C3 ribosyltransferase inhibits IL-2 production and sustained calcium influx in activated T cells. J. Immunol. 163, 3819–3825 (1999).
Samelson, L. E., Phillips, A. F., Luong, E. T. & Klausner, R. D. Association of the Fyn protein-tyrosine kinase with the T-cell antigen receptor. Proc. Natl Acad. Sci. USA 87, 4358–4362 (1990).
Krause, M. et al. Fyn-binding protein (Fyb)/SLP-76-associated protein (SLAP), Ena/Vasodilator-stimulated prosphoprotein (VASP) proteins and the Arp2/3 complex link T cell receptor (TCR) signaling to the actin cytoskeleton. J. Cell Biol. 149, 181–194 (2000).
Stein, P. L., Lee, H. M., Rich, S. & Soriano, P. pp59Fyn mutant mice display differential signaling in thymocytes and peripheral T cells. Cell 70, 741–750 (1992).
Vasioukhin, V., Bauer, C., Yin, M. & Fuchs, E. Directed actin polymerization is the driving force for epithelial cell- cell adhesion. Cell 100, 209–219 (2000).
Negulescu, P. A., Kraieva, T., Khan, A., Kerschbaum, H. H. & Cahalan, M. D. Polarity of T cell shape, motility, and sensitivity to antigen. Immunity 4, 421–430 (1996).
Dustin, M. L., Bromely, S. K., Kan, Z., Peterson, D. A. & Unanue, E. R. Antigen receptor engagement delivers a stop signal to migrating T lymphocytes. Proc. Natl Acad. Sci. USA 94, 3909–3913 (1997).
Iezzi, G., Karjalainen, K. & Lanzavecchia, A. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity 8, 89–95 (1998).
Lowin-Kropf, B., Shapiro, V. S. & Weiss, A. Cytoskeletal polarization of T cells is regulated by an immunoreceptor tyrosine-based activation motif-dependent mechanism. J. Cell Biol. 140, 861–871 (1998).
Kersh, E. N., Shaw, A. S. & Allen, P. M. Fidelity of T cell activation through multistep T cell receptor zeta phosphorylation. Science 281, 572–575 (1998).
Chan, A. C., Desai, D. M. & Weiss, A. The role of protein tyrosine kinases and protein tyrosine phopshatases in T cell antigen receptor signal transduction. Annu. Rev. Immunol. 12, 555–592 (1994).
Plas, D. R. et al. Direct regulation of ZAP-70 by SHP-1 in T cell antigen receptor signaling. Science 272, 1173–1176 (1996).
Mustelin, T. et al. Regulation of the p70zap tyrosine protein kinase in T cells by the CD45 phosphotyrosine phosphatase. Eur. J. Immunol. 25, 942–946 (1995).
Joazeiro, C. A. et al. The tyrosine kinase negative regulator c-Cbl as a RING-type, E2-dependent ubiquitin-protein ligase. Science 286, 309–312 (1999).
Levkowitz, G. et al. Ubiquitin ligase activity and tyrosine phosphorylation underlie suppression of growth factor signaling by c-Cbl/Sli-1. Mol. Cell 4, 1029–1040 (1999).
Keane, M. M., Rivero-Lezcano, O. M., Mitchell, J. A., Robbins, K. C. & Lipkowitz, S. Cloning and characterization of cbl-b: a SH3 binding protein with homology to the c-cbl proto-oncogene. Oncogene 10, 2367–2377 (1995).
Bachmaier, K. et al. Negative regulation of lymphocyte activation and autoimmunity by the molecular adaptor Cbl-b. Nature 403, 211–216 (2000).
Wardenburg, J. B. et al. Phosphorylation of SLP-76 by the ZAP-70 protein-tyrosine kinase is required for T-cell receptor function. J. Biol. Chem. 271, 19641–19644 (1996).
Crespo, P., Schuebel, K. E., Ostrom, A. A., Gutkind, J. S. & Bustelo, X. R. Phosphotyrosine-dependent activation of Rac-1 GDP/GTP exchange by the vav proto-oncogene product. Nature 385, 169–172 (1997).
Raab, M., da Silva, A. J., Findell, P. R. & Rudd, C. E. Regulation of Vav-SLP-76 binding by ZAP-70 and its relevance to TCR ζ/CD3 induction of interleukin-2. Immunity 6, 155–164 (1997).
Zhang, W., Sloan-Lancaster, J., Kitchen, J., Trible, R. P. & Samelson, L. E. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell 92, 83–92 (1998).
Kim, A. S., Kakalis, L. T., Abdul-Manan, N., Liu, G. A. & Rosen, M. K. Autoinhibition and activation mechanisms of the Wiskott-Aldrich syndrome protein. Nature 404, 151–158 (2000).
Blanchoin, L. et al. Direct observation of dendritic actin filament networks nucleated by Arp2/3 complex and WASp/Scar proteins. Nature 404, 1007–1011 (2000).
Ramesh, N., Anton, I. M., Hartwig, J. H. & Geha, R. S. WIP, a protein associated with wiskott-aldrich syndrome protein, induces actin polymerization and redistribution in lymphoid cells. Proc. Natl Acad. Sci. USA 94, 14671–14676 (1997).
Frischknecht, F. et al. Actin-based motility of vaccinia virus mimics receptor tyrosine kinase signalling. Nature 401, 926–929 (1999).
Tilney, L. G. & Portnoy, D. A. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J. Cell. Biol. 109, 1597–1608 (1989).
Welch, M. D., Rosenblatt, J., Skoble, J., Portnoy, D. A. & Mitchison, T. J. Interaction of human Arp2/3 complex and the Listeria monocytogenes ActA protein in actin filament nucleation. Science 281, 105–108 (1998).
Loisel, T. P., Boujemaa, R., Pantaloni, D. & Carlier, M. F. Reconstitution of actin-based motility of Listeria and Shigella using pure proteins . Nature 401, 613–616 (1999).
Cooper, J. A. & Schafer, D. A. Control of actin assembly and disassembly at filament ends. Curr. Opin. Cell Biol. 12, 97–103 (2000).
Mullins, R. D., Heuser, J. A. & Pollard, T. D. The interaction of Arp2/3 complex with actin: nucleation, high affinity pointed end capping, and formation of branching networks of filaments. Proc. Natl Acad. Sci. USA 95, 6181–6186 (1998).
Snapper, S. B. et al. Wiskott-Aldrich syndrome protein-deficient mice reveal a role for WASp in T but not B cell activation. Immunity 9, 81–91 (1998).
Snapper, S. B. & Rosen, F. S. The Wiskott-Aldrich syndrome protein (WASp): roles in signaling and cytoskeletal organization. Annu. Rev. Immunol. 17, 905–929 (1999).
Bresnick, A. R. Molecular mechanisms of nonmuscle myosin-II regulation. Curr. Opin. Cell Biol. 11, 26–33 (1999).
Svitkina, T. M., Verkhovsky, A. B., McQuade, K. M. & Borisy, G. G. Analysis of the actin-myosin II system in fish epidermal keratocytes: mechanism of cell body translocation. J. Cell Biol. 139, 397–415 (1997).
Verkhovsky, A. B., Svitkina, T. M. & Borisy, G. G. Self-polarization and directional motility of cytoplasm. Curr. Biol. 9, 11–20 (1999).
Verkhovsky, A. B., Svitkina, T. M. & Borisy, G. G. Network contraction model for cell translocation and retrograde flow. Biochem. Soc. Symp. 65, 207–222 (1999).
Edwards, D. C., Sanders, L. C., Bokoch, G. M. & Gill, G. N. Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nat. Cell Biol. 1, 253–259 (1999).
Dustin, M. L. et al. A novel adapter protein orchestrates receptor patterning and cytoskeletal polarity in T cell contacts. Cell 94, 667–677 (1998).
Shih, N. Y. et al. Congenital Nephrotic Syndrome in Mice Lacking CD2-Associated Protein. Science 286, 312–315 (1999).
Paul, W. E. & Seder, R. A. Lymphocyte responses and cytokines. Cell 76, 241–251 (1994).
Valututti, S., Müller, S., Cella, M., Padovan, E. & Lanzavecchia, A. Serial triggering of many T-cell receptors by a few peptide-MHC complexes. Nature 375, 148–151 (1995).
Delon, J. et al. CD8 expression allows T cell signaling by monomeric peptide-MHC complexes. Immunity 9, 467–473 (1998).
van der Merwe, P. A., Davis, S. J., Shaw, A. S. & Dustin, M. L. Cytoskeletal polarization and redistribution of cell-surface molecules during T cell antigen recognition. Semin. Immunol. 12, 5–21 (2000).
Sykulev, Y., Joo, M., Vturina, I., Tsomides, T. J. & Eisen, H. N. Evidence that a single peptide-MHC complex on a target cell can elicit a cytolytic T cell response. Immunity 4, 565–571 (1996).
Underhill, D. M., Bassetti, M., Rudensky, A. & Aderem, A. Dynamic interactions of macrophages with T cells during antigen presentation. J. Exp. Med. 190, 1909–1914 (1999).
Cherry, R. J. et al. Detection of dimers of dimers of human leukocyte antigen (HLA)-Dr on surface of living cells by single-particle fluorescence imaging. J. Cell Biol. 140, 71–79 (1998).
Vale, R. D. et al. Direct observation of single kinesin molecules moving along microtubules. Nature 380, 451–453 (1996).
Lanzavecchia, A. Understanding the mechanism of sustained signaling and T cell activation. J. Exp. Med. 185, 1717–1719 (1997).
Viola, A. & Lanzavecchia, A. T cell activation determined by T cell receptor number and tunable thresholds. Science 273, 104–106 (1996).
Ernst, B., Lee, D. S., Chang, J. M., Sprent, J. & Surh, C. D. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity 11, 173–181 (1999).
Yasutomo, K., Doyle, C., Miele, L. & Germain, R. N. The duration of antigen receptor signalng determines CD4+ versus CD8+ T-cell lineage fate. Nature 404, 506–510 (2000).
Sun, Z. et al. PKC-θ is required for TCR-induced NK-κB activation in mature but not immature T lymphocytes. Nature 404, 402–407 (2000).
Monks, C. R., Kupfer, H., Tamir, I., Barlow, A. & Kupfer, A. Selective modulation of protein kinase C-theta during T-cell activation. Nature 385, 83–86 (1997).
Dianzani, U., Shaw, A., Al-Ramadi, B. K., Kubo, R. T. & Janeway, C. A. Physical association of CD4 with the T cell receptor. J. Immunol. 148, 678–688 (1992).
Klauck, T. M. et al. Coordination of three signaling enzymes by AKAP79, a mammalian scaffold protein. Science 271, 1589–1592 (1996).
Nauert, J. B., Klauck, T. M., Langeberg, L. K. & Scott, J. D. Gravin, an autoantigen recognized by serum from myasthenia gravis patients, is a kinase scaffold protein. Curr. Biol. 7, 52–62 (1997).
Ingulli, E., Mondino, A., Khoruts, A. & Jenkins, M. K. In vivo detection of dendritic cell antigen presentation to CD4(+) T cells. J. Exp. Med. 185, 2133–2141 (1997).
Garside, P. et al. Visualization of specific B and T lymphocyte interactions in the lymph node. Science 281, 96–99 (1998).
Frolov, V. A. I. I. Mechnikoff's contribution to immunology. J. Hyg. Epidemiol. Microbiol. Immunol. 29, 185–191 (1985).
Huang, J. F. et al. TCR-Mediated internalization of peptide-MHC complexes acquired by T cells. Science 286, 952–954 (1999).
Acknowledgements
We thank D. Schafer, K. Blumer and A. Shaw for their suggestions and comments. M.L.D. and J.A.C. are supported by grants from the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Movie 1.
Initial distribution of the T cell receptor. T cell receptors tagged with Alexa-488 H57 Fab were imaged by confocal microscope (30 x time lapse). Note the non homogeneous distribution of the TCR on the surface and the evidence of membrane dynamics. Images captured with Shannon Bromely, Kenneth Johnson and John Heuser.
Movie 2.
Formation of an immunological synapse. T cell receptors were tagged with Alexa 488-H57 Fab and live cells were imaged on a confocal microscope (30 x time lapse). This 3A9 TCR transgenic T cell just came into contact with an Hen Egg White Lysozyme pulsed C3F6 B lymphoma cell (APC). The first frame is a bright feild image showing the T cell at the lower right and the B cell at the upper left. The movie spans a period of 8 minutes in 20 seconds of video. By the end of the movie the TCR has moved to the center of the contact area. Some reduction in total fluorescence is attributable to photobleaching. Images captured with Shannon Bromely, Kenneth Johnson and John Heuser.
Movie 3.
Three dimensional rendering of early time point from movie 2.
Movie 4.
Three dimensional rendering of late time point from movie 2.
Movie 5.
Integrin mediated sensory contact. Interference reflection image of a T cell contact with a serum coated supported planar bilayer. Image is shown in real time.
Movie 6.
Contact area dynamics in immunological synapse. T cell reacting with planar bialyer containing MHC-peptide complex and serum proteins (integrin ligands). Note membrane ruffling at the periphery of the immunological synapse. Image is shown in real time. Images captured with Cenk Sumen.
Movie 7.
Contact area dynamics triggered by TCR engagement alone. T cell reacting with planar bilayer containing MHC-peptide complex only. Note the profound membrane dynamics, which may reflect the stimulation of action polymerization by transiently engaged TCR. The contact has a dynamic and lacy appearance. Image is shown in real time. Images captured with Chistophe Wülfing.
Rights and permissions
About this article
Cite this article
Dustin, M., Cooper, J. The immunological synapse and the actin cytoskeleton: molecular hardware for T cell signaling. Nat Immunol 1, 23–29 (2000). https://doi.org/10.1038/76877
Issue Date:
DOI: https://doi.org/10.1038/76877
This article is cited by
-
Relapse of acute myeloid leukemia after allogeneic stem cell transplantation: immune escape mechanisms and current implications for therapy
Molecular Cancer (2023)
-
Essential function of adaptor protein Nck1 in platelet-derived growth factor receptor signaling in human lens epithelial cells
Scientific Reports (2022)
-
Virus interactions with the actin cytoskeleton—what we know and do not know about SARS-CoV-2
Archives of Virology (2022)
-
The chaperonin CCT8 controls proteostasis essential for T cell maturation, selection, and function
Communications Biology (2021)
-
Modulation of lung cytoskeletal remodeling, RXR based metabolic cascades and inflammation to achieve redox homeostasis during extended exposures to lowered pO2
Apoptosis (2021)