Abstract
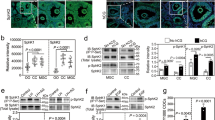
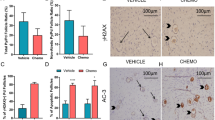
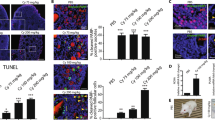
The time at which ovarian failure (menopause) occurs in females is determined by the size of the oocyte reserve provided at birth, as well as by the rate at which this endowment is depleted throughout post-natal life. Here we show that disruption of the gene for acid sphingomyelinase in female mice suppressed the normal apoptotic deletion of fetal oocytes, leading to neonatal ovarian hyperplasia. Ex vivo, oocytes lacking the gene for acid sphingomyelinase or wild-type oocytes treated with sphingosine-1-phosphate resisted developmental apoptosis and apoptosis induced by anti-cancer therapy, confirming cell autonomy of the death defect. Moreover, radiation-induced oocyte loss in adult wild-type female mice, the event that drives premature ovarian failure and infertility in female cancer patients, was completely prevented by in vivo therapy with sphingosine-1-phosphate. Thus, the sphingomyelin pathway regulates developmental death of oocytes, and sphingosine-1-phosphate provides a new approach to preserve ovarian function in vivo.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Gougeon, A. Regulation of ovarian follicular development in primates: facts and hypotheses . Endocr. Rev. 17, 121– 155 (1996).
Baker, T.G. A quantitative and cytological study of germ cells in human ovaries. Proc. R. Soc. Lond. (B) 158, 417–433 (1963).
Perez, G.I. et al. Prolongation of ovarian lifespan into advanced chronological age by Bax-deficiency. Nature Genet. 21, 200–203 (1999).
Morita, Y. & Tilly J.L. Oocyte apoptosis: like sand through an hourglass. Dev. Biol. 213, 1–17 (1999).
Gosden, G.G & Faddy, M.J. Biological basis of premature ovarian failure. Reprod. Fertil. Dev. 10, 73–78 (1998).
Hammond, C.B. Menopause and hormone replacement therapy: an overview. Obstet. Gynecol. 87, 2S–15S ( 1996).
Waxman, J. Chemotherapy and the adult gonad: a review. J. Royal Soc. Med. 76, 144–148 ( 1983).
Ried, H.L. & Jaffe, N. Radiation-induced changes in long-term survivors of childhood cancer after treatment with radiation therapy. Sem. Roentgenol. 29, 6– 14 (1994).
Blumenfeld, Z., Avivi, I., Ritter, M. & Rowe, J.M. Preservation of fertility and ovarian function and minimizing chemotherapy-induced gonadotoxicity in young women. J. Soc. Gynecol. Investig. 6, 229–239 (1999).
Reynolds, T. Cell death genes may hold clues to preserving fertility after chemotherapy . J. Natl. Cancer Inst. 91, 664– 666 (1999).
Rudin, C.M. & Thompson, C.B. Apoptosis and disease: regulation and clinical relevance of programmed cell death. Annu. Rev. Med. 48, 267–281 ( 1997).
Thornberry, N.A. & Lazebnik, Y. Caspases: enemies within. Science 281, 1312– 1316 (1998).
Cryns, V. & Yuan, J. Proteases to die for. Genes Dev. 12, 1551–1570 (1998).
Xiang, J., Chao, D.T. & Korsmeyer, S.J. BAX-induced cell death may not require interleukin-1β-converting enzyme-like proteases. Proc. Natl. Acad. Sci. USA 93 , 14559–14563 (1996).
Kolesnick, R.N. & Kronke, M. Regulation of ceramide production and apoptosis. Annu. Rev. Physiol. 60, 643–665 (1998).
Spiegel, S. et al. Sphingosine-1-phosphate in cell growth and death. Ann. N.Y. Acad. Sci. 845, 11–18 (1998).
Olivera, A. et al. Sphingosine kinase expression increases intracellular sphingosine-1-phosphate and promotes cell growth and survival. J. Cell Biol. 147, 545–558 (1999).
Cuvillier, O. et al. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature 381, 800 –803 (1996).
Horinouchi, K. et al. Acid sphingomyelinase deficient mice: a model of types A and B Niemann-Pick disease. Nature Genet. 10, 288–293 (1995).
Morita, Y. et al. Requirement for phosphatidylinositol-3′-kinase in cytokine-mediated germ cell survival during fetal oogenesis in the mouse. Endocrinology 140, 941–949 ( 1999).
Wang, E., Norred, W.P., Bacon, C.W., Riley, R.T. & Merrill, A.H. Inhibition of sphingolipid biosynthesis by fumonisins. J. Biol. Chem. 266, 14486 –14490 (1991).
Bose, R. et al. Ceramide synthase mediates daunorubicin-induced apoptosis: an alternative mechanism for generating death signals. Cell 82, 405–414 (1995).
Perez, G.I., Tao, X.-J. & Tilly, J.L. Fragmentation and death (a.k.a. apoptosis) of ovulated oocytes. Mol. Hum. Reprod. 5, 414–420 (1999).
Van Brocklyn, J.R. et al. Dual actions of sphingosine-1-phosphate: extracellular through the Gi-coupled receptor Edg-1 and intracellular to regulate proliferation and survival. J. Cell Biol. 142, 229– 240 (1998).
Van Brocklyn, J.R., Tu, Z., Edsall, L.C., Schmidt, R.R. & Spiegel, S. Sphingosine 1-phosphate-induced cell rounding and neurite retraction are mediated by the G protein-coupled receptor H218. J. Biol. Chem. 274, 4626– 4232 (1999).
Edsall, L.C., Pirianov, G.G. & Spiegel, S. Involvement of sphingosine 1-phosphate in nerve growth factor-mediated neuronal survival and differentiation. J. Neurosci. 17, 6952–6960 ( 1997).
Goetzl, E.J. & An, S. A subfamily of G protein-coupled cellular receptors for lysophospholipids and lysosphingolipids. Adv. Exp. Med. Biol. 469, 259–264 (1999).
Hla, T. et al. Sphingosine-1-phosphate: extracellular mediator or intracellular second messenger? Biochem. Pharmacol. 58, 201–207 (1999).
Lee, M.J. et al. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell 99, 301–312 (1999).
Dong, J. et al. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature 383, 531– 535 (1996).
Gosden, R.G. The vocabulary of the egg. Nature 383, 485 –486 (1996).
Jurisicova, A., Varmuza, S. & Casper, R.F. Involvement of programmed cell death in preimplantation embryo demise. Hum. Reprod. Update 1, 558–566 (1995).
Hardy, K. Apoptosis in the human embryo. Rev. Reprod. 4, 125–134 (1999).
Perez, G.I., Trbovich, A.M., Gosden R.G. & Tilly, J.L. Mitochondria and the death of oocytes. Nature 403, 500–501 (2000).
Ratts, V.S., Flaws, J.A., Kolp, R., Sorenson, C.M. & Tilly, J.L. Ablation of bcl-2 gene expression decreases the number of oocytes and primordial follicles established in the post-natal female mouse gonad. Endocrinology 136, 3665–3668 (1995).
Perez, G.I., Knudson, C.M., Leykin, L., Korsmeyer, S.J. & Tilly, J.L. Apoptosis-associated signaling pathways are required for chemotherapy-mediated female germ cell destruction. Nature Med. 3, 1228– 1332 (1997).
Morita, Y., Perez, G.I., Maravei, D.V., Tilly, K.I. & Tilly, J.L. Targeted expression of Bcl-2 in mouse oocytes inhibits ovarian follicle atresia and prevents spontaneous and chemotherapy-induced oocyte apoptosis in vitro. Mol. Endocrinol. 13, 841–850 ( 1999).
Bergeron, L. et al. Defects in regulation of apoptosis in caspase-2-deficient mice. Genes Dev. 12, 1304– 1314 (1998).
Ding, H.F. & Fisher, D.E. Mechanisms of p53-mediated apoptosis. Crit. Rev. Oncog. 9, 83– 98 (1998).
Cuvillier, O., Rosenthal, D.S., Smulson, M.E. & Spiegel, S. Sphingosine 1-phosphate inhibits activation of caspases that cleave poly(ADP-ribose) polymerase and lamins during Fas- and ceramide-mediated apoptosis in Jurkat T lymphocytes. J. Biol. Chem. 273 , 2910–2916 (1998).
Pastorino, J.G. et al. Functional consequences of the sustained or transient activation by Bax of the mitochondrial permeability transition pore. J. Biol. Chem. 274, 31734–31739 ( 1999).
Lantz, M.E. in Cancer Obstetrics and Gynecology (eds. Trimble, E.L. & Trimble, C.L.) 87–98 (Lippincott Williams and Wilkins, Philadelphia, 1999).
Morita, Y. & Tilly, J.L. Segregation of retinoic acid effects on fetal ovarian germ cell mitosis versus apoptosis by requirement for new macromolecular synthesis. Endocrinology 140 , 2696–2703 (1999).
He, X. et al. Characterization of human acid sphingomyelinase purified from the media of overexpressing Chinese hamster ovary cells. Biochim. Biophys. Acta 1432, 251–264 ( 1999).
Jürgensmeier, J.M. et al. Bax directly induces release of cytochrome c from isolated mitochondria. Proc. Natl. Acad. Sci. USA 95, 4997–5002 (1998).
Fairbairn, D.W., Olive, P.L. & O'Neill, K.L. The comet assay: a comprehensive overview . Mutat. Res. 339, 37–59 (1995).
Acknowledgements
The authors thank I. Schiff for discussions after his critical review of the manuscript before its submission, and S. Riley for technical assistance with the image analysis and data presentation. This study was supported by National Institutes of Health grants R01-AG12279 (J.L.T.), R01-HD34226 (J.L.T.), R01-ES08430 (J.L.T.) and R01- CA423852 (R.N.K.), and by Vincent Memorial Research Funds (J.L.T.). This work was done while Y.M. was on leave from the Department of OB/GYN of the University of Tokyo Faculty of Medicine and supported by the Japanese Society for the Promotion of Science, and while G.I.P. was supported in part by a grant from the Harvard Center of Excellence in Women's Health.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morita, Y., Perez, G., Paris, F. et al. Oocyte apoptosis is suppressed by disruption of the acid sphingomyelinase gene or by sphingosine -1-phosphate therapy. Nat Med 6, 1109–1114 (2000). https://doi.org/10.1038/80442
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/80442
This article is cited by
-
Roles of follicle stimulating hormone and sphingosine 1-phosphate co-administered in the process in mouse ovarian vitrification and transplantation
Journal of Ovarian Research (2023)
-
An intestinal sphingolipid confers intergenerational neuroprotection
Nature Cell Biology (2023)
-
Thyroid hormone triiodothyronine does not protect ovarian reserve from DNA damage induced by X-ray and cisplatin
Journal of Assisted Reproduction and Genetics (2023)
-
TrkB agonist antibody ameliorates fertility deficits in aged and cyclophosphamide-induced premature ovarian failure model mice
Nature Communications (2022)
-
Egg yolk lipids: separation, characterization, and utilization
Food Science and Biotechnology (2022)