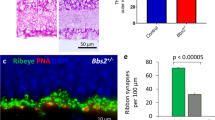
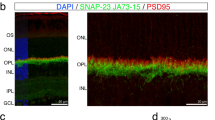
Abstract
In addition to rod photoreceptor loss, many mutations in rod photoreceptor-specific genes cause degeneration of other neuronal types. Identifying mechanisms of cell–cell interactions initiated by rod-specific mutations and affecting other retinal cells is important for understanding the pathogenesis and progression of retinal degeneration. Here we show in animals with rod and cone degeneration due to mutations in the genes encoding rhodopsin and cGMP phosphodiesterase β-subunit (PDE-β) respectively, that rod bipolar cells received ectopic synapses from cones in the absence of rods. Thus, synaptic plasticity links certain rod-specific mutations to retina-wide structural alterations that involve different types of neurons.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Rattner, A., Sun, H. & Nathans, J. Molecular genetics of human retinal disease. Annu. Rev. Genet. 33, 89–131 (1999).
Berson, E. L. Retinitis pigmentosa. The Friedenwald Lecture. Invest. Ophthalmol. Vis. Sci. 34, 1659–1676 (1993).
Dryja, T. P. & Berson, E. L. Retinitis pigmentosa and allied diseases: implications of genetic heterogeneity. Invest. Ophthalmol. Vis. Sci. 36, 1197–1200 (1995).
Bird, A. C. Retinal photoreceptor dystrophies. Am. J. Ophthalmol. 119, 543–562 (1995).
Gal, A., Apfelstedt-Sylla, E., Janecke, A. R. & Zrenner, E. Rhodopsin mutations in inherited retinal dystrophies and dysfunctions. Prog. Ret. Eye Res. 16, 51–79 (1997).
Santos, A. et al. Preservation of the inner retina in retinitis pigmentosa: a morphometric analysis. Arch. Ophthalmol. 115, 511–515 (1997).
Humayun, M. S. et al. Morphometric analysis of the extramacular retina from postmortem eyes with retinitis pigmentosa. Invest. Ophthalmol. Vis. Sci. 40, 143–148 (1999).
Cideciyan, A. V. & Jacobson, S. G. Negative electroretinograms in retinitis pigmentosa. Invest. Ophthalmol. Vis. Sci. 34, 3253–3263 (1993).
Sieving, P. A. Photopic ON- and OFF-pathway abnormalities in retinal dystrophies. Trans. Am. Ophthalmol. Soc. 91, 701–773 (1993).
Chang, G. Q., Hao, Y. & Wong, F. Apoptosis: final common pathway of photoreceptor death in rd, rds, and rhodopsin mutant mice. Neuron 11, 595–605 (1993).
Portera-Cailliau, C., Sung, C.-H., Nathans, J. & Adler, R. Apoptotic photoreceptor cell death in mouse models of retinitis pigmentosa. Proc. Natl. Acad. Sci. USA 91, 974–978 (1994).
Lolley, R. N., Rong, H. & Craft, C. M. Linkage of photoreceptor degeneration by apoptosis with inherited defect in phototransduction. Invest. Ophthalmol. Vis. Sci. 35, 358–362 (1994).
Huang, P. C., Gaitan, A. E., Hao, Y., Petters, R. M. & Wong, F. Cellular interactions implicated in the mechanism of photoreceptor degeneration in transgenic mice expressing a mutant rhodopsin gene. Proc. Natl. Acad. Sci. USA 90, 8484–8488 (1993).
Kedzierski, W., Bok, D. & Tavis, G. H. Non-cell autonomous photoreceptor degeneration in rds mutant mice mosaic for expression of a rescue transgene. J. Neurosci. 18, 4076–4082 (1998).
Petters, R. M. et al. Genetically engineered large animal model for studying cone photoreceptor survival and degeneration in retinitis pigmentosa. Nat. Biotechnol. 15, 965–970 (1997).
Farber, D. B., Flannery, J. G. & Bowes-Rickman, C. . The rd mouse story: Seventy years of research on an animal model of inherited retinal degeneration. Prog. Retinal Eye Res. 13, 31–64 (1994).
Wässle, H. & Boycott, B. B. Functional architecture of the mammalian retina. Physiol. Rev. 71, 447–480 (1991).
Sterling, P. in The Synaptic Organization of the Brain (ed. Shepherd, G. M.) 205–253 (Oxford Univ. Press, New York, 1998).
Sharp, L. T. & Stockman, A. Rod pathways: the importance of seeing nothing. Trends Neurosci. 22, 497–504 (1999).
Dowling, J. E. The Retina: an Approachable Part of the Brain (Harvard Univ. Press, Cambridge, Massachusetts, 1987).
Negishi, K., Kato, S. & Teranishi, T. Dopamine cells and rod bipolar cells contain protein kinase C-like immunoreactivity in some vertebrate retinas. Neurosci. Lett. 94, 247–253 (1988).
Greferath, U., Grunert, U. & Wässle, H. Rod bipolar cells in the mammalian retina show protein kinase C-like immunoreactivity. J. Comp. Neurol. 301, 433–442 (1990).
Wässle, H., Yamashita, M., Greferath, U., Grunert, U. & Muller, F. The rod bipolar cell of the mammalian retina. Vis. Neurosci. 7, 99–112 (1991).
Grunert, U., Martin, P. R. & Wässle, H. Immunocytochemical analysis of bipolar cells in the macaque monkey retina. J. Comp. Neurol. 348, 607–627 (1994).
Wässle, H., Grunert, U. & Rohrenbeck, J. Immunocytochemical staining of AII-amacrine cells in the rat retina with antibodies against parvalbumin. J. Comp. Neurol. 332, 407–420 (1993).
Casini, G., Rickman, D. W. & Brecha, N. C. AII amacrine cell population in the rabbit retina: identification by parvalbumin immunoreactivity. J. Comp. Neurol. 356, 132–142 (1995).
Brandstatter, J. H., Lohrke, S., Morgans, C. W. & Wässle, H. Distributions of two homologous synaptic vesicle proteins, synaptoporin and synaptophysin, in the mammalian retina. J. Comp. Neurol. 370, 1–10 (1996).
Ong, O. C., et al. Molecular cloning and characterization of the G protein gamma subunit of cone photoreceptors. J. Biol. Chem. 270, 8495–8500 (1995).
Masu, M. et al. Specific deficit of the ON response in visual transmission by targeted disruption of the mGluR6 gene. Cell 80, 757–765 (1995).
Vardi, N. Alpha subunit of Go localizes in the dendritic tips of ON bipolar cells. J. Comp. Neurol. 395, 43–52 (1998).
Brandstatter, J. H., Koulen, P. & Wässle, H. Diversity of glutamate receptors in the mammalian retina. Vision Res. 38, 1385–1397 (1998).
Peichl, L. & Bolz, J. Kainic acid induces sprouting of retinal neurons. Science 223, 503–504 (1984).
Lewis, G. P., Linberg, K. A. & Fisher, S. K. Neurite outgrowth from bipolar and horizontal cells after experimental retinal detachment. Invest. Ophthalmol. Vis. Sci. 39, 424–434 (1998).
Cowan, W. M., Fawcett, J. W., O'Leary, D. D. & Stanfield, B. B. Regressive events in neurogenesis. Science 225, 1258–1265 (1984).
Soucy, E., Wang, Y., Nirenberg, S., Nathans, J. & Meister, M. A novel signaling pathway from rod photoreceptor to ganglion cells in mammalian retina. Neuron 21, 481–493 (1998).
Ying, S., Jansen, H. T., Lehman, M. N., Fong, S. L. & Kao, W. Y. Retinal degeneration in cone photoreceptor cell-ablated transgenic mice. Mol. Vis. 6, 101–108 (2000).
Blanks, J. C., Adinolfi, A. M. & Lolley, R. N. Synaptogenesis in the photoreceptor terminal of the mouse retina. J. Comp. Neurol. 156, 81–94 (1974).
Blanks, J. C., Adinolfi, A. M. & Lolley, R. N. Photoreceptor degeneration and synaptogenesis in retinal-degenerative (rd) mice. J. Comp. Neurol. 156, 95–106 (1974).
Li, Z-Y., Kljavin, I. J. & Milam, A. H. Rod photoreceptor neurite sprouting in retinitis pigmentosa. J. Neurosci. 15, 5429–5438 (1995).
Fariss, R. N., Li, Z. Y. & Milam, A. H. Abnormalities in rod photoreceptors, amacrine cells and horizontal cells in human retinas with retinitis pigmentosa. Am. J. Ophthal. 129, 215–223 (2000).
Allikmets, R. et al. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat. Genet. 15, 236–246 (1997).
Sun, H. & Nathans, J. Stargardt's ABCR is localized to the disc membrane of retinal rod outer segments. Nat. Genet. 17, 15–16 (1997).
Weng, J. et al. Insights into the function of Rim protein in photoreceptors and etiology of Stargardt's disease from the phenotype in abcr knockout mice. Cell 98, 13–23 (1999).
Illing, M., Molday, L. L. & Molday, R. S. The 220-kDa rim protein of retinal rod outer segments is a member of the ABC transporter superfamily. J. Biol. Chem. 272, 10303–10310 (1997).
Sun, H., Molday, R. S. & Nathans, J. Retinal stimulates ATP hydrolysis by purified and reconstituted ABCR, the photoreceptor-specific ATP-binding cassette transporter responsible for Stargardt disease. J. Biol. Chem. 274, 8269–8281 (1999).
Banin, E. et al. Retinal rod photoreceptor-specific gene mutation perturbs cone pathway development. Neuron 23, 549–557 (1999).
Lam, B. L., Liu, M. & Hamasaki, D. I. Low-frequency damped electroretinographic wavelets in young asymptomatic patients with dominant retinitis pigmentosa. Ophthalmology 106, 1109–1113 (1999).
Berson, E. L., Gouras, P., Gunkel, R. D. & Myrianthopoulos, N. C. Dominant retinitis pigmentosa with reduced penetrance. Arch. Opthalmol. 81, 226–234 (1969).
Berson, E. L. & Simonoff, E. A. Dominant retinitis pigmentosa with reduced penetrance. Arch. Ophthalmol. 97, 1286–1291 (1979).
Acknowledgements
We thank members of the North Carolina State University Swine Educational Unit for producing the pigs, Bruce Collins and Jeffrey Sommer for genotyping pigs, Ewa Worniallo for assistance with electron microscopy and Paul Hargrave of the University of Florida for the anti-rhodopsin mouse monoclonal antibody. This work was supported by the National Eye Institute (RO1EY11498 and P30EY05722), Foundation Fighting Blindness of Hunt Valley, Maryland and Research to Prevent Blindness of New York, New York.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peng, YW., Hao, Y., Petters, R. et al. Ectopic synaptogenesis in the mammalian retina caused by rod photoreceptor-specific mutations. Nat Neurosci 3, 1121–1127 (2000). https://doi.org/10.1038/80639
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/80639
This article is cited by
-
The temporal progression of retinal degeneration and early-stage idebenone treatment in the Pde6brd1/rd1 mouse model of retinal dystrophy
Scientific Reports (2024)
-
Molecular basis of retinal remodeling in a zebrafish model of retinitis pigmentosa
Cellular and Molecular Life Sciences (2023)
-
Mechanism of Cone Degeneration in Retinitis Pigmentosa
Cellular and Molecular Neurobiology (2023)
-
Nr2e3 is a genetic modifier that rescues retinal degeneration and promotes homeostasis in multiple models of retinitis pigmentosa
Gene Therapy (2021)
-
Management of cystoid macular edema secondary to retinitis pigmentosa via subliminal micropulse yellow laser
Lasers in Medical Science (2021)