Abstract
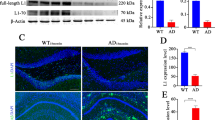
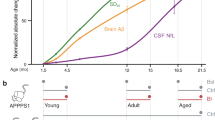
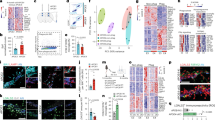
Abnormal accumulation of the amyloid-β peptide (Aβ) in the brain appears crucial to pathogenesis in all forms of Alzheimer disease (AD), but the underlying mechanisms in the sporadic forms of AD remain unknown. Transforming growth factor β1 (TGF-β1), a key regulator of the brain's responses to injury and inflammation, has been implicated in Aβ deposition in vivo. Here we demonstrate that a modest increase in astroglial TGF-β1 production in aged transgenic mice expressing the human β-amyloid precursor protein (hAPP) results in a three-fold reduction in the number of parenchymal amyloid plaques, a 50% reduction in the overall Aβ load in the hippocampus and neocortex, and a decrease in the number of dystrophic neurites. In mice expressing hAPP and TGF-β1, Aβ accumulated substantially in cerebral blood vessels, but not in parenchymal plaques. In human cases of AD, Aβ immunoreactivity associated with parenchymal plaques was inversely correlated with Aβ in blood vessels and cortical TGF-β1 mRNA levels. The reduction of parenchymal plaques in hAPP/TGF-β1 mice was associated with a strong activation of microglia and an increase in inflammatory mediators. Recombinant TGF-β1 stimulated Aβ clearance in microglial cell cultures. These results demonstrate that TGF-β1 is an important modifier of amyloid deposition in vivo and indicate that TGF-β1 might promote microglial processes that inhibit the accumulation of Aβ in the brain parenchyma.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Selkoe, D.J. Translating cell biology into therapeutic advances in Alzheimer's disease. Nature 399, A23–A31 (1999).
Terry, R.D., Katzman, R., Bick, K.L. & Sisodia, S.S. Alzheimer Disease. 2nd edn. (Lippincott Williams & Wilkins, Philadelphia, 1999).
Games, D. et al. Alzheimer-type neuropathology in transgenic mice overexpressing V717F β-amyloid precursor protein. Nature 373, 523–527 (1995).
Hsiao, K. et al. Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science 274, 99–102 (1996).
Sturchler-Pierrat, C. et al. Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc. Natl. Acad. Sci. USA 94, 13287–13292 (1997).
Nicoll, J.A.R., Roberts, G.W. & Graham, D.I. Apolipoprotein E ɛ4 allele is associated with deposition of amyloid β-protein following head injury. Nature Med. 1, 135–137 (1995).
Guo, Z. et al. Head injury and the risk of AD in the MIRAGE study. Neurology 54, 1316–1323 (2000).
Zlokovic, B.V. Cerebrovascular transport of Alzheimer's amyloid β and apolipoproteins J and E: Possible anti-amyloidogenic role of the blood-brain barrier. Life Sci. 59, 1483–1497 (1996).
Bales, K.R. et al. Lack of apolipoprotein E dramatically reduces amyloid β-peptide deposition. Nat. Genet. 17, 263–264 (1997).
Holtzman, D.M. et al. Expression of human apolipoprotein E reduces amyloid-β deposition in a mouse model of Alzheimer's disease. J. Clin. Invest. 103, R15–R21 (1999).
Frautschy, S.A., Cole, G.M. & Baird, A. Phagocytosis and deposition of vascular β-amyloid in rat brains injected with Alzheimer β-amyloid. Am. J. Pathol. 140, 1389–1399 (1992).
Qiu, W.Q. et al. Insulin-degrading enzyme regulates extracellular levels of amyloid β-protein by degradation. J. Biol. Chem. 273, 32730–32738 (1998).
Yan, S.D., Roher, A., Schmidt, A.M. & Stern, D.M. Cellular cofactors for amyloid β-peptide-induced cell stress. Moving from cell culture to in vivo. Am. J. Pathol. 155, 1403–1411 (1999).
Schenk, D. et al. Immunization with amyloid-β attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature 400, 173–177 (1999).
Bard, F. et al. Peripherally administered antibodies against amyloid β-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nature Med. 6, 916–919 (2000).
Giulian, D. Microglia and the immune pathology of Alzheimer disease. Am. J. Hum. Genet. 65, 13–18 (1999).
Frackowiak, J. et al. Ultrastructure of the microglia that phagocytose amyloid and the microglia that produce β-amyloid fibrils. Acta Neuropathol. 84, 225–233 (1992).
Finch, C.E., Laping, N.J., Morgan, T.E., Nichols, N.R. & Pasinetti, G.M. TGF-β1 is an organizer of responses to neurodegeneration. J. Cell. Biochem. 53, 314–322 (1993).
Wyss-Coray, T. et al. Amyloidogenic role of cytokine TGF-β1 in transgenic mice and Alzheimer's disease. Nature 389, 603–606 (1997).
Frautschy, S.A., Yang, F., Calderón, L. & Cole, G. M. Rodent models of Alzheimer's disease: Rat Aβ infusion approaches to amyloid deposits. Neurobiol. Aging 17, 311–321 (1996).
Flanders, K.C., Ren, R.F. & Lippa, C.F. Transforming growth factor-βs in neurodegenerative disease. Prog. Neurobiol. 54, 71–85 (1998).
Akiyama, H. et al. Inflammation and Alzheimer's disease. Neuroinflammation Working Group. Neurobiol. Aging 21, 383–421 (2000).
Roberts, A.B. & Sporn, M.B. Transforming growth factor-β, in The Molecular and Cellular Biology of Wound Repair 2nd edn. (ed. Clark, R.A.F.), 275–308 (Plenum, New York, 1996).
Wyss-Coray, T. et al. Increased central nervous system production of extracellular matrix components and development of hydrocephalus in transgenic mice overexpressing transforming growth factor-β1. Am. J. Pathol. 147, 53–67 (1995).
Wyss-Coray, T., Lin, C., Sanan, D., Mucke, L. & Masliah, E. Chronic overproduction of TGF-β1 in astrocytes promotes Alzheimer's disease-like microvascular degeneration in transgenic mice. Am. J. Pathol. 156, 139–150 (2000).
Wahl, S.M. Transforming growth factor beta (TGF-β) in inflammation: A cause and a cure. J. Clin. Immunol. 12, 61–74 (1992).
Yao, J., Harvath, L., Gilbert, D. & Colton, C. Chemotaxis by a CNS macrophage, the microglia. J. Neurosci. Res. 27, 36–42 (1990).
Wyss-Coray, T., Borrow, P., Brooker, M.J. & Mucke, L. Astroglial overproduction of TGF-β1 enhances inflammatory central nervous system disease in transgenic mice. J. Neuroimmunol. 77, 45–50 (1997).
Hsia, A. et al. Plaque-independent disruption of neural circuits in Alzheimer's disease mouse models. Proc. Natl. Acad. Sci. USA 96, 3228–3233 (1999).
Mucke, L. et al. High-level neuronal expression of Aβ1–42 in wild-type human amyloid protein precursor transgenic mice: Synaptotoxicity without plaque formation. J. Neurosci. 20, 4050–4058 (2000).
Barelli, H. et al. Characterization of new polyclonal antibodies specific for 40 and 42 amino acid-long amyloid β peptides: Their use to examine the cell biology of presenilins and the immunohistochemistry of sporadic Alzheimer's disease and cerebral amyloid angiopathy cases. Mol. Med. 3, 695–707 (1997).
Johnson-Wood, K. et al. Amyloid precursor protein processing and Aβ42 deposition in a transgenic mouse model of Alzheimer disease. Proc. Natl. Acad. Sci. USA 94, 1550–1555 (1997).
Masliah, E. et al. Comparison of neurodegenerative pathology in transgenic mice overexpressing V717F β-amyloid precursor protein and Alzheimer's disease. J. Neurosci. 16, 5795–5811 (1996).
Paxinos, G. The Rat Nervous System. 2nd edn. (Academic, San Diego, 1995).
Mann, D.M. et al. Predominant deposition of amyloid-β 42(43) in plaques in cases of Alzheimer's disease and hereditary cerebral hemorrhage associated with mutations in the amyloid precursor protein gene. Am. J. Pathol. 148, 1257–1266 (1996).
Kuo, Y.-M. et al. Comparative analysis of Aβ chemical structure and amyloid plaque morphology of transgenic mice and alzheimer disease brains. J. Biol. Chem. (in the press).
Weller, R.O. et al. Cerebral amyloid angiopathy. Amyloid β accumulates in putative interstitial fluid drainage pathways in Alzheimer's disease. Am. J. Pathol. 153, 725–733 (1998).
Border, W.A. & Noble, N.A. TGF-β in kidney fibrosis: A target for gene therapy. Kidney Int. 51, 1388–1396 (1997).
Galbreath, E., Kim, S.-J., Park, K., Brenner, M. & Messing, A. Overexpression of TGF-β1 in the central nervous system of transgenic mice results in hydrocephalus. J. Neuropathol. Exp. Neurol. 54, 339–349 (1995).
Kisilevsky, R. Proteoglycans and other basement membrane proteins in amyloidoses. Mol. Neurobiol. 9, 23–24 (1994).
Calhoun, M.E. et al. Neuronal overexpression of mutant amyloid precursor protein results in prominent deposition of cerebrovascular amyloid. Proc. Natl. Acad. Sci. USA 96, 14088–14093 (1999).
Verbeek, M.M., Otte-Höller, I., Veerhuis, R., Ruiter, D.J. & De Waal, R.M.W. Distribution of Aβ-associated proteins in cerebrovascular amyloid of Alzheimer's disease. Acta. Neuropathol. 96, 628–636 (1998).
McGeer, P.L. & McGeer, E.G. Inflammation of the brain in Alzheimer's disease: Implications for therapy. J. Leukocyte Biol. 65, 409–415 (1999).
Olichney, J.M. et al. The apolipoprotein E4 allele is associated with increased neuritic plaques and cerebral amyloid angiopathy in Alzheimer's disease and Lewy body variant. Neurology 47, 190–196 (1996).
Acknowledgements
We thank G. Howard and S. Ordway for editorial assistance; N. Nemenzo for secretarial help; and J.C.W. Carroll, C. Goodfellow and S. Gonzales for graphics and photography. This work was supported by National Institutes of Health Grants AG-15871 (T.W.-C.), AG-5131 and AG-10689 (E.M.), AG-11385 (L.M.), and by the Alzheimer's Association (T.W.-C.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wyss-Coray, T., Lin, C., Yan, F. et al. TGF-β1 promotes microglial amyloid-β clearance and reduces plaque burden in transgenic mice. Nat Med 7, 612–618 (2001). https://doi.org/10.1038/87945
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/87945
This article is cited by
-
Pathogenesis of Depression in Alzheimer’s Disease
Neurochemical Research (2024)
-
Clinical evidence of human pathogens implicated in Alzheimer’s disease pathology and the therapeutic efficacy of antimicrobials: an overview
Translational Neurodegeneration (2023)
-
TGF-β1 signalling in Alzheimer’s pathology and cytoskeletal reorganization: a specialized Tau perspective
Journal of Neuroinflammation (2023)
-
Perivascular cells induce microglial phagocytic states and synaptic engulfment via SPP1 in mouse models of Alzheimer’s disease
Nature Neuroscience (2023)
-
Vγ1 and Vγ4 gamma-delta T cells play opposing roles in the immunopathology of traumatic brain injury in males
Nature Communications (2023)