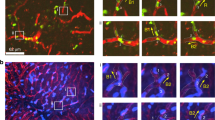
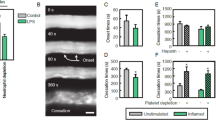
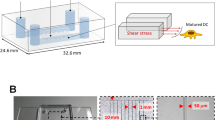
Abstract
Leukocyte transendothelial migration (TEM) is thought to be a chemotactic process controlled by chemokine gradients across the endothelium. Using cytokine-activated human umbilical vascular endothelial cells (HUVECs) as a model of inflamed endothelium, we have shown that apical endothelial chemokines can trigger robust peripheral blood lymphocyte (PBL) migration across endothelial cells. Lymphocyte TEM was promoted by physiological shear stress applied continuously to migrating lymphocytes. Lymphocyte integrins, intact actin cytoskeleton and Gi protein–mediated chemokine signaling, but not a chemotactic gradient, were mandatory for TEM. PBL TEM did not require intracellular free calcium or intact phosphatidyl inositol kinase activity in migrating lymphocytes. Thus, lymphocyte TEM is promoted by fluid shear-induced mechanical signals coupled to Gi protein signals at apical endothelial zones.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Campbell, J. J. & Butcher, E. C. Chemokines in tissue-specific and microenvironment-specific lymphocyte homing. Curr. Opin. Immunol. 12, 336–341 (2000).
Mackay, C. R. Chemokines: immunology's high impact factors. Nature Immunol. 2, 95–101 (2001).
Springer, T. A. Traffic signals for lymphocyte recirculation and leukocyte emigration: The multistep paradigm. Cell 76, 301–314 (1994).
Butcher, E. C. & Picker, L. J. Lymphocyte homing and homeostasis. Science 272, 60–66 (1996).
Bargatze, R. F. & Butcher, E. C. Rapid G protein-regulated activation event involved in lymphocyte binding to high endothelial venules. J. Exp. Med. 178, 367–372 (1993).
Campbell, J. J., Hedrick, J., Zlotnik, A., Siani, M. A. & Thompson, D. A. Chemokines and the arrest of lymphocytes rolling under flow conditions. Science 279, 381–384 (1998).
Grabovsky, V. et al. Sub-second Induction of integrin clustering by immobilized chemokines enhances leukocyte capture and rolling under flow prior to firm adhesion to endothelium. J. Exp. Med. 192, 495–505 (2000).
Bianchi, E., Bender, J. R., Blasi, F. & Pardi, R. Through and beyond the wall: late steps in leukocyte transendothelial migration. Immunol. Today 18, 586–591 (1997).
Weber, K. S., von Hundelshausen, P., Clark-Lewis, I., Weber, P. C. & Weber, C. Differential immobilization and hierarchical involvement of chemokines in monocyte arrest and transmigration on inflamed endothelium in shear flow. Eur. J. Immunol. 29, 700–712 (1999).
Allport, J. R., Muller, W. A. & Luscinskas, F. W. Monocytes induce reversible focal changes in vascular endothelial cadherin complex during transendothelial migration under flow. J. Cell Biol. 148, 203–216 (1999).
Johnson-Leger, C., Aurrand-Lions, M. & Imhof, B. A. The parting of the endothelium: miracle, or simply a junctional affair? J. Cell Sci. 113, 921–933 (2000).
Shang, X. Z. & Issekutz, A. C. Contribution of CD11a/CD18, CD11b/CD18, ICAM-1 (CD54) and -2 (CD102) to human monocyte migration through endothelium and connective tissue fibroblast barriers. Eur. J. Immunol. 28, 1970–1979 (1998).
Ding, Z. et al. Regulation of chemokine-induced transendothelial migration of T lymphocytes by endothelial activation: differential effects on naive and memory T cells. J. Leuko. Biol. 67, 825–833 (2000).
Middleton, J. et al. Transcytosis and surface presentation of IL-8 by venular endothelial cells. Cell 91, 385–395 (1997).
Piali, L. et al. The chemokine receptor CXCR3 mediates rapid and shear-resistant adhesion-induction of effector T lymphocytes by the chemokines IP10 and Mig. Eur. J. Immunol. 28, 961–972 (1998).
Campbell, J. J. et al. The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature 400, 776–780 (1999).
Pablos, J. L. et al. Stromal-cell derived factor is expressed by dendritic cells and endothelium in human skin. Am. J. Pathol. 155, 1577–1586 (1999).
Peled, A. et al. The chemokine SDF-1 stimulates integrin-mediated arrest of CD34(+) cells on vascular endothelium under shear flow. J. Clin. Invest. 104, 1199–1211 (1999).
Stein, J. V. et al. The CC chemokine thymus-derived chemotactic agent 4 (TCA-4, secondary lymphoid tissue chemokine, 6Ckine, Exodus-2) triggers lymphocyte function-associated antigen 1-mediated arrest of rolling T lymphocytes in peripheral lymph node high endothelial venules. J. Exp. Med. 191, 61–76 (2000).
Luscinskas, F. W. et al. L- and P-selectins, but not CD49d (VLA-4) integrins, mediate monocyte initial attachment to TNF-α-activated vascular endothelium under flow in vitro. J. Immunol. 157, 326–335 (1996).
Su, W. H., Chen, H., Huang, J. & Jen, C. J. Endothelial [Ca2+]i signaling during transmigration of polymorphonuclear leukocytes. Blood 96, 3816–3822 (2000).
Gupta, S. K., Lysko, P. G., Pillarisetti, K., Ohlstein, E. & Stadel, J. M. Chemokine receptors in human endothelial cells. Functional expression of CXCR4 and its transcriptional regulation by inflammatory cytokines. J. Biol. Chem. 273, 4282–4287 (1998).
Luu, N. T., Rainger, G. E. & Nash, G. B. Differential ability of exogenous chemotactic agents to disrupt transendothelial migration of flowing neutrophils. J. Immunol. 164, 5961–5969 (2000).
Kuijpers, T. W. et al. Freezing adhesion molecules in a state of high-avidity binding blocks eosinophil migration. J. Exp. Med. 178, 279–284 (1993).
Del Maschio, A. et al. Polymorphonuclear leukocyte adhesion triggers the disorganization of endothelial cell-to-cell adherens junctions. J. Cell. Biol. 135, 497–510 (1996).
Andriopoulou, P., Navarro, P., Zanetti, A., Lampugnani, M. G. & Dejana, E. Histamine induces tyrosine phosphorylation of endothelial cell-to-cell adherens junctions. Arterioscler. Thromb. Vasc. Biol. 19, 2286–2297 (1999).
Candal, F. J. et al. BMEC-1: a human bone marrow microvascular endothelial cell line with primary cell characteristics. Microvasc. Res. 52, 221–234 (1996).
Honda, S. et al. Ligand-induced adhesion to activated endothelium and to vascular cell adhesion molecule-1 in lymphocytes transfected with the N-formyl peptide receptor. J. Immunol. 152, 4026–4035 (1994).
Eddy, R. J., Pierini, L. M., Matsumura, F. & Maxfield, F. R. Ca2+-dependent myosin II activation is required for uropod retraction during neutrophil migration. J. Cell Sci. 113, 1287–1298. (2000).
Thelen, M. Dancing to the tune of chemokines. Nature Immunol. 2, 129–134 (2001).
Hendey, B., Klee, C. B. & Maxfield, F. R. Inhibition of neutrophil chemokinesis on vitronectin by inhibitors of calcineurin. Science 258, 296–299 (1992).
Lawson, M. A. & Maxfield, F. R. Ca(2+)- and calcineurin-dependent recycling of an integrin to the front of migrating neutrophils. Nature 377, 75–79 (1995).
Sanchez-Madrid, F. & del Pozo, M. A. Leukocyte polarization in cell migration and immune interactions. EMBO J. 18, 501–511 (1999).
Hirsch, E. et al. Central role for G protein-coupled phosphoinositide 3-kinase γ in inflammation. Science 287, 1049–1053 (2000).
Servant, G. et al. Polarization of chemoattractant receptor signaling during neutrophil chemotaxis. Science 287, 1037–1040 (2000).
Moazzam, F., DeLano, F. A., Zweifach, B. W. & Schmid-Schonbein, G. W. The leukocyte response to fluid stress. Proc. Natl Acad. Sci. USA 94, 5338–5343 (1997).
Okuyama, M., Ohta, Y., Kambayashi, J. I. & Monden, M. Fluid shear stress induces actin polymerization in human neutrophils. J. Cell. Biochem. 63, 432–441 (1996).
Li, S. et al. Fluid shear stress activation of focal adhesion kinase. Linking to mitogen-activated protein kinases. J. Biol. Chem. 272, 30455–30462 (1997).
Manolopoulos, V. G. et al. Inhibition of angiogenesis by blockers of volume-regulated anion channels. Gen. Pharmacol. 34, 107–116 (2000).
Yamamoto, K., Korenaga, R., Kamiya, A. & Ando, J. Fluid shear stress activates Ca(2+) influx into human endothelial cells via P2X4 purinoceptors. Circ. Res. 87, 385–391 (2000).
Gotsch, U. et al. VE-cadherin antibody accelerates neutrophil recruitment in vivo. J. Cell Sci. 110, 583–588 (1997).
Huang, A. J. et al. Endothelial cell cytosolic free calcium regulates neutrophil migration across monolayers of endothelial cells. J. Cell Biol. 120, 1371–1380 (1993).
Tinsley, J. H., Wu, M. H., Ma, W., Taulman, A. C. & Yuan, S. Y. Activated neutrophils induce hypersensitivity and phosphorylation of adherens junction proteins in coronary venular endothelial cells. J. Biol. Chem. 274, 24930–24934 (1999).
Levenberg, S., Katz, B. Z., Yamada, K. M. & Geiger, B. Long-range and selective autoregulation of cell-cell or cell-matrix adhesions by cadherin or integrin ligands. J. Cell Sci. 111, 347–357 (1998).
Burns, A. R. et al. Analysis of tight junctions during neutrophil transendothelial migration. Neutrophil transendothelial migration is independent of tight junctions and occurs preferentially at tricellular corners. J. Cell Sci. 113, 45–57 (2000).
Poznansky, M. C. et al. Active movement of T cells away from a chemokine. Nature Med. 6, 543–548 (2000).
Utgaard, J. O., Jahnsen, F. L., Bakka, A., Brandtzaeg, P. & Haraldsen, G. Rapid secretion of prestored interleukin 8 from Weibel-Palade bodies of microvascular endothelial cells. J. Exp. Med. 188, 1751–1756 (1998).
Kawai, T. et al. Selective diapedesis of Th1 cells induced by endothelial cell RANTES. J. Immunol. 163, 3269–3278 (1999).
Carr, M. W., Alon, R. & Springer, T. A. The C-C chemokine MCP-1 differentially modulates the avidity of β1 and β2 integrins on T lymphocytes. Immunity 4, 179–187 (1996).
Jaffe, E. A., Nachman, N. L., Becker, C. J. & Minick, C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J. Clin. Invest. 52, 2745–2756 (1973).
Dwir, O. et al. GlyCAM-1 Supports Leukocyte Tethering and Rolling: evidence for a greater dynamic stability of L-selectin rolling of lymphocytes than of neutrophils. Cell Adhesion Commun. 6, 349–370 (1998).
Hemler, M. E. et al. Glycoproteins of 210,000 and 130,000 m.w. on activated T cells: cell distribution and antigenic relation to components on resting cells and T cell lines. J. Immunol. 132, 3011–3018 (1984).
Andrew, D. et al. KIM185, a monoclonal antibody to CD18 which induces a change in the conformation of CD18 and promotes both LFA-1- and CR3-dependent adhesion. Eur. J. Immunol. 23, 2217–2222 (1993).
Shinder, V., Amir, R. & Devor, M. Cross-excitation in dorsal root ganglia does not depend on close cell-to-cell apposition. Neuroreport 9, 3997–4000 (1998).
Acknowledgements
We thank M. Lipp, R. Lobb and S. Raffi, for providing reagents and S. Shwarzbaum for editorial help. Special thanks to S. Feigelson and A. Peled for helpful discussions. Supported in part by the Israel Science Foundation and the Minnerva Foundation, Germany.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Web Movie 1.
Digitized Quicktime videos showing characteristic scenes from time-lapse recordings of lymphocytes accumulated on TNF-α-activated HUVEC monolayers overlaid with SDF-1a (100 ng/ml) and subjected to continuous shear stress of 5 dyn/cm2. The time (in min) elapsed from the beginning of the shear application phase is shown at the top. Shown is a magnified image of a 290x200 micron field, selected from full 540x400 micron field (described in Methods). Lymphocytes are numbered from 1–10 to allow monitoring of their position throughout the assay. Arrows point to cells 6 and 8, which are in the process of TEM. Lymphocyte number 7 undergoes TEM at t=2.55 min. The remaining lymphocytes locomote over the surface without transmigrating through the ECs. (MOV 5476 kb)
Web Movie 2.
Digitized Quicktime videos showing characteristic scenes from timelapse recordings of lymphocytes accumulated on TNF-α-activated HUVEC monolayers overlaid with SDF-1α (100 ng/ml) and subjected to shear-free conditions. The time (in min) elapsed from the beginning of the shear application phase is shown at the top. For further details refer to the legend of Web Movie 1. No lymphocyte TEM occurs under these experimental conditions. (MOV 5306 kb)
Rights and permissions
About this article
Cite this article
Cinamon, G., Shinder, V. & Alon, R. Shear forces promote lymphocyte migration across vascular endothelium bearing apical chemokines. Nat Immunol 2, 515–522 (2001). https://doi.org/10.1038/88710
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/88710
This article is cited by
-
Immune cell behaviour and dynamics in the kidney — insights from in vivo imaging
Nature Reviews Nephrology (2022)
-
Co-receptor signaling in the pathogenesis of neuroHIV
Retrovirology (2021)
-
In-vitro-Methoden zur Untersuchung von Scherstress auf Zellen
Gefässchirurgie (2021)
-
Identification and local manipulation of bone marrow vasculature during intravital imaging
Scientific Reports (2020)
-
Photopic light-mediated down-regulation of local α1A-adrenergic signaling protects blood-retina barrier in experimental autoimmune uveoretinitis
Scientific Reports (2019)