Abstract
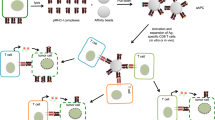
Primary tumor cells genetically modified to express a collection of immunological ligands on their surface may have the utility as therapeutic autologous cancer vaccines. However, genetic modification of primary tumor cells is not only cost, labor and time intensive, but also has safety repercussions. As an alternative, we developed the ProtEx technology that involves generation of immunological ligands with core streptavidin (SA) and their display on biotinylated cells in a rapid and efficient manner. We herein demonstrate that TC-1 tumor cells can be rapidly and efficiently engineered to codisplay on their surface two costimulatory proteins, SA-4-1BBL and SA-LIGHT, simultaneously. Vaccination with irradiated TC-1 cells codisplaying both chimeric proteins showed 100% efficacy in a prophylactic and >55% efficacy in a therapeutic tumor setting. In contrast, vaccination with TC-1 cells engineered with either protein alone showed significantly reduced efficacy in the prophylactic setting. Vaccine efficacy was associated with the generation of primary and memory T-cell and antibody responses against the tumor without detectable signs of autoimmunity. Engineering tumor cells in a rapid and effective manner to simultaneously display on their surface a collection of immunostimulatory proteins with additive/synergistic functions presents a novel alternative approach to gene therapy with considerable potential for cancer immunotherapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Croft M . The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol 2009; 9: 271–285.
Anderson RC, Anderson DE, Elder JB, Brown MD, Mandigo CE, Parsa AT et al. Lack of B7 expression, not human leukocyte antigen expression, facilitates immune evasion by human malignant gliomas. Neurosurgery 2007; 60: 1129–1136.
Gimmi CD, Freeman GJ, Gribben JG, Gray G, Nadler LM . Human T-cell clonal anergy is induced by antigen presentation in the absence of B7 costimulation. Proc Natl Acad Sci USA 1993; 90: 6586–6590.
van de Corput L, Falkenburg JH, Kester MG, Willemze R, Kluin-Nelemans JC . Impaired expression of CD28 on T cells in hairy cell leukemia. Clin Immunol 1999; 93: 256–262.
Townsend SE, Allison JP . Tumor rejection after direct costimulation of CD8+ T cells by B7-transfected melanoma cells. Science 1993; 259: 368–370.
Bai XF, Bender J, Liu J, Zhang H, Wang Y, Li O et al. Local costimulation reinvigorates tumor-specific cytolytic T lymphocytes for experimental therapy in mice with large tumor burdens. J Immunol 2001; 167: 3936–3943.
Ramarathinam L, Castle M, Wu Y, Liu Y . T cell costimulation by B7/BB1 induces CD8 T cell-dependent tumor rejection: an important role of B7/BB1 in the induction, recruitment, and effector function of antitumor T cells. J Exp Med 1994; 179: 1205–1214.
Townsend SE, Allison JP . Tumor rejection after direct costimulation of CD8+ T cells by B7- transfected melanoma cells. Science 1993; 259: 368–370.
Cornetta K, Morgan RA, Anderson WF . Safety issues related to retroviral-mediated gene transfer in humans. Hum Gene Ther 1991; 2: 5–14.
Pfeifer A, Verma IM . Gene therapy: promises and problems. Annu Rev Genomics Hum Genet 2001; 2: 177–211.
Anderson WF . Gene therapy. The best of times, the worst of times. Science 2000; 288: 627–629.
Yolcu ES, Askenasy N, Singh NP, Cherradi SE, Shirwan H . Cell membrane modification for rapid display of proteins as a novel means of immunomodulation: FasL-decorated cells prevent islet graft rejection. Immunity 2002; 17: 795–808.
Pahler A, Hendrickson WA, Kolks MA, Argarana CE, Cantor CR . Characterization and crystallization of core streptavidin. J Biol Chem 1987; 262: 13933–13937.
Sharma RK, Elpek KG, Yolcu ES, Schabowsky R-H, Zhao H, Bandura-Morgan L et al. Costimulation as a platform for the development of vaccines: a peptide-based vaccine containing a novel from of 4-1BBL eradicates established tumors. Cancer Res 2009; 69: 4319–4326.
Elpek KG, Yolcu ES, Franke DD, Lacelle C, Schabowsky R-H, Shirwan H . Ex vivo expansion of CD4+CD25+FoxP3+ T regulatory cells based on synergy between IL-2 and 4-1BB signaling. J Immunol 2007; 179: 7295–7304.
Kilinc MO, Mukundan L, Yolcu ES, Singh NP, Suttles J, Shirwan H . Generation of a multimeric form of CD40L with potent immunostimulatory activity using streptavidin as a chaperon. Exp Mol Pathol 2006; 80: 252–261.
Singh NP, Miller RW, Yolcu ES, Kilinc MO, Oechsli M, Huseby R et al. Primary tumor cells from cancer patients decorated with a novel form of CD80 protein serve as effective antigen-presenting cells for the induction of autologous T-cell immune responses ex vivo. Hum Gene Ther 2006; 17: 334–346.
Singh NP, Yolcu ES, Taylor DD, Gercel-Taylor C, Metzinger DS, Dreisbach SK et al. A novel approach to cancer immunotherapy: tumor cells decorated with CD80 generate effective antitumor immunity. Cancer Res 2003; 63: 4067–4073.
Guinn BA, DeBenedette MA, Watts TH, Berinstein NL . 4-1BBL cooperates with B7-1 and B7-2 in converting a B cell lymphoma cell line into a long-lasting antitumor vaccine. J Immunol 1999; 162: 5003–5010.
Johnson BD, Gershan JA, Natalia N, Zujewski H, Weber JJ, Yan X et al. Neuroblastoma cells transiently transfected to simultaneously express the co-stimulatory molecules CD54, CD80, CD86, and CD137L generate antitumor immunity in mice. J Immunother 2005; 28: 449–460.
Bertram EM, Lau P, Watts TH . Temporal segregation of 4-1BB versus CD28-mediated costimulation: 4-1BB ligand influences T cell numbers late in the primary response and regulates the size of the T cell memory response following influenza infection. J Immunol 2002; 168: 3777–3785.
Wilcox RA, Chapoval AI, Gorski KS, Otsuji M, Shin T, Flies DB et al. Cutting edge: expression of functional CD137 receptor by dendritic cells. J Immunol 2002; 168: 4262–4267.
Melero I, Shuford WW, Newby SA, Aruffo A, Ledbetter JA, Hellström KE et al. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat Med 1997; 3: 682–685.
Murillo O, Arina A, Hervas-Stubbs S, Gupta A, McCluskey B, Dubrot J et al. Therapeutic antitumor efficacy of anti-CD137 agonistic monoclonal antibody in mouse models of myeloma. Clin Cancer Res 2008; 14: 6895–6906.
Uno T, Takeda K, Kojima Y, Yoshizawa H, Akiba H, Mittler RS et al. Eradication of established tumors in mice by a combination antibody-based therapy. Nat Med 2006; 12: 693–698.
Wilcox RA, Tamada K, Flies DB, Zhu G, Chapoval AI, Blazar BR et al. Ligation of CD137 receptor prevents and reverses established anergy of CD8+ cytolytic T lymphocytes in vivo. Blood 2004; 103: 177–184.
Wilcox RA, Flies DB, Zhu G, Johnson AJ, Tamada K, Chapoval AI et al. Provision of antigen and CD137 signaling breaks immunological ignorance, promoting regression of poorly immunogenic tumors. J Clin Invest 2002; 109: 651–659.
Fan Z, Yu P, Wang Y, Fu ML, Liu W, Sun Y et al. NK-cell activation by LIGHT triggers tumor-specific CD8+ T-cell immunity to reject established tumors. Blood 2006; 107: 1342–1351.
Yu P, Lee Y, Liu W, Wang J, Wang Y, Schietinger A et al. Priming of naive T cells inside tumors leads to eradication of established tumors. Nat Immunol 2004; 5: 141–149.
Zhai Y, Guo R, Hsu TL, Yu GL, Ni J, Kwon BS et al. LIGHT, a novel ligand for lymphotoxin beta receptor and TR2/HVEM induces apoptosis and suppresses in vivo tumor formation via gene transfer. J Clin Invest 1998; 102: 1142–1151.
Xu G, Liu D, Okwor I, Wang Y, Korner H, Kung SK et al. LIGHT Is critical for IL-12 production by dendritic cells, optimal CD4+ Th1 cell response, and resistance to Leishmania major. J Immunol 2007; 179: 6901–6909.
Elpek KG, Lacelle C, Singh NP, Yolcu ES, Shirwan H . CD4+CD25+ T regulatory cells dominate multiple immune evasion mechanisms in early but not late phases of tumor development in a B cell lymphoma model. J Immunol 2007; 178: 6840–6848.
Mauri DN, Ebner R, Montgomery RI, Kochel KD, Cheung TC, Yu GL et al. LIGHT, a new member of the TNF superfamily, and lymphotoxin alpha are ligands for herpesvirus entry mediator. Immunity 1998; 8: 21–30.
Yu P, Lee Y, Liu W, Chin RK, Wang J, Wang Y et al. Priming of naive T cells inside tumors leads to eradication of established tumors. Nat Immunol 2004; 5: 141–149.
Guinn BA, DeBenedette MA, Watts TH, Berinstein NL . 4-1BBL cooperates with B7-1 and B7-2 in converting a B cell lymphoma cell line into a long-lasting antitumor vaccine. J Immunol 1999; 162: 5003–5010.
Johnson BD, Gershan JA, Natalia N, Zujewski H, Weber JJ, Yan X et al. Neuroblastoma cells transiently transfected to simultaneously express the co-stimulatory molecules CD54, CD80, CD86, and CD137L generate antitumor immunity in mice. J Immunother 2005; 28: 449–460.
Sotomayor EM, Borrello I, Rattis FM, Cuenca AG, Abrams J, Staveley-O'Carroll K et al. Cross-presentation of tumor antigens by bone marrow-derived antigen-presenting cells is the dominant mechanism in the induction of T-cell tolerance during B-cell lymphoma progression. Blood 2001; 98: 1070–1077.
Dennis JW, Donaghue TP, Kerbel RS . An examination of tumor antigen loss in spontaneous metastases. Invasion Metastasis 1981; 1: 111–125.
Giorgi JV, Burton RC, Scott D, Warner NL . Tumor immunity to murine plasma cell tumors. VII. Expression of H-2 and tumor antigens on Ig synthesis variants of MPC-11. Int J Cancer 1982; 29: 119–126.
Guckel B, Stumm S, Rentzsch C, Marme A, Mannhardt G, Wallwiener D . A CD80-transfected human breast cancer cell variant induces HER-2/neu-specific T cells in HLA-A*02-matched situations in vitro as well as in vivo. Cancer Immunol Immunother 2005; 54: 129–140.
Yolcu ES, Gu X, Lacelle C, Zhao H, Bandura-Morgan L, Askenasy N et al. Induction of tolerance to cardiac allografts using donor splenocytes engineered to display on their surface an exogenous fas ligand protein. J Immunol 2008; 181: 931–939.
Pollok KE, Kim YJ, Zhou Z, Hurtado J, Kim KK, Pickard RT et al. Inducible T cell antigen 4-1BB. Analysis of expression and function. J Immunol 1993; 150: 771–781.
Montgomery RI, Warner MS, Lum BJ, Spear PG . Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell 1996; 87: 427–436.
Tamada K, Shimozaki K, Chapoval AI, Zhu G, Sica G, Flies D et al. Modulation of T-cell-mediated immunity in tumor and graft-versus-host disease models through the LIGHT co-stimulatory pathway. Nat Med 2000; 6: 283–289.
Morel Y, Truneh A, Sweet RW, Olive D, Costello RT . The TNF superfamily members LIGHT and CD154 (CD40 ligand) costimulate induction of dendritic cell maturation and elicit specific CTL activity. J Immunol 2001; 167: 2479–2486.
Ware CF . Targeting lymphocyte activation through the lymphotoxin and LIGHT pathways. Immunol Rev 2008; 223: 186–201.
Heo SK, Ju SA, Lee SC, Park SM, Choe SY, Kwon B et al. LIGHT enhances the bactericidal activity of human monocytes and neutrophils via HVEM. J Leukoc Biol 2006; 79: 330–338.
Yu P, Fu YX . Targeting tumors with LIGHT to generate metastasis-clearing immunity. Cytokine Growth Factor Rev 2008; 19: 285–294.
Zhang N, Sadun RE, Arias RS, Flanagan ML, Sachsman SM, Nien YC et al. Targeted and untargeted CD137L fusion proteins for the immunotherapy of experimental solid tumors. Clin Cancer Res 2007; 13: 2758–2767.
Disis ML, Scholler N, Dahlin A, Pullman J, Knutson KL, Hellström KE et al. Plasmid-based vaccines encoding rat neu and immune stimulatory molecules can elicit rat neu-specific immunity. Mol Cancer Ther 2003; 2: 995–1002.
Zou GM, Martinson J, Hu WY, Tam Y, Klingemann HG . The effect of LIGHT in inducing maturation of monocyte-derived dendritic cells from MDS patients. Cancer Immunol Immunother 2004; 53: 681–689.
Acknowledgements
We thank O Grimany for his technical assistance in proteins production and purification. This work was funded, in part, by grants from the NIH (R43 AI071618, R41 CA121665, R44 AI071618 and R43AI074176), the Kentucky Lung Cancer Research Program, WM Keck Foundation and the Commonwealth of Kentucky Research Challenge Trust Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The SA-4-1BBL described in this paper is licensed from UofL by ApoImmune, Inc, Louisville, KY, for which Haval Shirwan serves as CSO, and Haval Shirwan and Esma S Yolcu have significant equity interest in the Company. The remaining authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Sharma, R., Yolcu, E., Elpek, K. et al. Tumor cells engineered to codisplay on their surface 4-1BBL and LIGHT costimulatory proteins as a novel vaccine approach for cancer immunotherapy. Cancer Gene Ther 17, 730–741 (2010). https://doi.org/10.1038/cgt.2010.29
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cgt.2010.29
Keywords
This article is cited by
-
A novel agonist of 4-1BB costimulatory receptor shows therapeutic efficacy against a tobacco carcinogen-induced lung cancer
Cancer Immunology, Immunotherapy (2023)
-
Tumor-derived granulocyte colony-stimulating factor diminishes efficacy of breast tumor cell vaccines
Breast Cancer Research (2018)