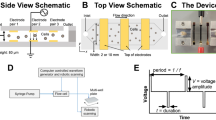
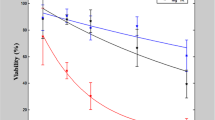
Abstract
Established cell transfection via nucleofection relies on nucleofection buffers with unknown and proprietary makeup due to trade secrecy, inhibiting the possibility of using this otherwise effective method for developing cell therapy. We devised a three-step method for discovering an optimal formulation for the nucleofection of any cell line. These steps include the selection of the best nucleofection program and known buffer type, selection of the best polymer for boosting the transfection efficiency of the best buffer and the comparison with the optimal buffer from an established commercial vendor (Amaxa). Using this three-step selection system, competitive nucleofection formulations were discovered for multiple cell lines, which are equal to or surpass the efficiency of the Amaxa nucleofector solution in a variety of cells and cell lines, including primary adipose stem cells, muscle cells, tumor cells and immune cells. Through the use of scanning electron microscopy, we have revealed morphological changes, which predispose for the ability of these buffers to assist in transferring plasmid DNA into the nuclear space. Our formulation may greatly reduce the cost of electroporation study in laboratory and boosts the potential of application of electroporation-based cell therapies in clinical trials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Pulendran B, Palucka K, Banchereau J . Sensing pathogens and tuning immune responses. Science 2001; 293: 253–256.
Paczesny S, Choi SW, Ferrara JL . Acute graft-versus-host disease: new treatment strategies. Curr Opin Hematol 2009; 16: 427–436.
Rappa G, Anzanello F, Alexeyev M, Fodstad O, Lorico A . Gamma-glutamylcysteine synthetase-based selection strategy for gene therapy of chronic granulomatous disease and graft-vs.-host disease. Eur J Haematol 2007; 78: 440–448.
Lee ST, Jang JH, Cheong JW, Kim JS, Maemg HY, Hahn JS et al. Treatment of high-risk acute myelogenous leukaemia by myeloablative chemoradiotherapy followed by co-infusion of T cell-depleted haematopoietic stem cells and culture-expanded marrow mesenchymal stem cells from a related donor with one fully mismatched human leucocyte antigen haplotype. Br J Haematol 2002; 118: 1128–1131.
Prata Kde L, Orellana MD, De Santis GC, Kashima S, Fontes AM, Carrara Rde C et al. Effects of high-dose chemotherapy on bone marrow multipotent mesenchymal stromal cells isolated from lymphoma patients. Exp Hematol 2010; 38: 292–300.e4.
Dupont KM, Sharma K, Stevens HY, Boerckel JD, Garcia AJ, Guldberg RE . Human stem cell delivery for treatment of large segmental bone defects. Proc Natl Acad Sci USA 2010; 107: 3305–3310.
Senju S, Hirata S, Motomura Y, Fukuma D, Matsunaga Y, Fukushima S et al. Pluripotent stem cells as source of dendritic cells for immune therapy. Int J Hematol 2010; 91: 392–400.
Katz AJ, Llull R, Hedrick MH, Futrell JW . Emerging approaches to the tissue engineering of fat. Clin Plast Surg 1999; 26: 587–603, viii.
Gimble J, Guilak F . Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy 2003; 5: 362–369.
Morizono K, De Ugarte DA, Zhu M, Zuk P, Elbarbary A, Ashjian P et al. Multilineage cells from adipose tissue as gene delivery vehicles. Hum Gene Ther 2003; 14: 59–66.
Josiah DT, Zhu D, Dreher F, Olson J, McFadden G, Caldas H . Adipose-derived stem cells as therapeutic delivery vehicles of an oncolytic virus for glioblastoma. Mol Ther 2010; 18: 377–385.
Zhang X, Godbey WT . Viral vectors for gene delivery in tissue engineering. Adv Drug Deliv Rev 2006; 58: 515–534.
Hendrie PC, Russell DW . Gene targeting with viral vectors. Mol Ther 2005; 12: 9–17.
Schaffer DV, Koerber JT, Lim KI . Molecular engineering of viral gene delivery vehicles. Annu Rev Biomed Eng 2008; 10: 169–194.
Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med 2006; 12: 342–347.
Howitt J, Anderson CW, Freimuth P . Adenovirus interaction with its cellular receptor CAR. Curr Top Microbiol Immunol 2003; 272: 331–364.
Strauss BE, Costanzi-Strauss E . Combating oncogene activation associated with retrovirus-mediated gene therapy of X-linked severe combined immunodeficiency. Braz J Med Biol Res 2007; 40: 601–613.
Check E . Gene therapy put on hold as third child develops cancer. Nature 2005; 433: 561.
Halama A, Kulinski M, Librowski T, Lochynski S . Polymer-based non-viral gene delivery as a concept for the treatment of cancer. Pharmacol Rep 2009; 61: 993–999.
Narang AS, Thoma L, Miller DD, Mahato RI . Cationic lipids with increased DNA binding affinity for nonviral gene transfer in dividing and nondividing cells. Bioconjug Chem 2005; 16: 156–168.
Bally MB, Harvie P, Wong FM, Kong S, Wasan EK, Reimer DL . Biological barriers to cellular delivery of lipid-based DNA carriers. Adv Drug Deliv Rev 1999; 38: 291–315.
Boussif O, Lezoualc’h F, Zanta MA, Mergny MD, Scherman D, Demeneix B et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci USA 1995; 92: 7297–7301.
Godbey WT, Wu KK, Mikos AG . Poly(ethylenimine)-mediated gene delivery affects endothelial cell function and viability. Biomaterials 2001; 22: 471–480.
Subramanian A, Ranganathan P, Diamond SL . Nuclear targeting peptide scaffolds for lipofection of nondividing mammalian cells. Nat Biotechnol 1999; 17: 873–877.
Mir LM . Nucleic acids electrotransfer-based gene therapy (electrogene therapy): past, current, and future. Mol Biotechnol 2009; 43: 167–176.
Neumann E, Schaefer-Ridder M, Wang Y, Hofschneider PH . Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J 1982; 1: 841–845.
Mir LM, Banoun H, Paoletti C . Introduction of definite amounts of nonpermeant molecules into living cells after electropermeabilization: direct access to the cytosol. Exp Cell Res 1988; 175: 15–25.
Cukjati D, Batiuskaite D, Andre F, Miklavcic D, Mir LM . Real time electroporation control for accurate and safe in vivo non-viral gene therapy. Bioelectrochemistry 2007; 70: 501–507.
Kotnik T, Mir LM, Flisar K, Puc M, Miklavcic D . Cell membrane electropermeabilization by symmetrical bipolar rectangular pulses. Part I. Increased efficiency of permeabilization. Bioelectrochemistry 2001; 54: 83–90.
Satkauskas S, Bureau MF, Puc M, Mahfoudi A, Scherman D, Miklavcic D et al. Mechanisms of in vivo DNA electrotransfer: respective contributions of cell electropermeabilization and DNA electrophoresis. Mol Ther 2002; 5: 133–140.
Jacobsen F, Mertens-Rill J, Beller J, Hirsch T, Daigeler A, Langer S et al. Nucleofection: a new method for cutaneous gene transfer? J Biomed Biotechnol 2006; 2006: 26060.
Maasho K, Marusina A, Reynolds NM, Coligan JE, Borrego F . Efficient gene transfer into the human natural killer cell line, NKL, using the Amaxa nucleofection system. J Immunol Methods 2004; 284: 133–140.
Trompeter HI, Weinhold S, Thiel C, Wernet P, Uhrberg M . Rapid and highly efficient gene transfer into natural killer cells by nucleofection. J Immunol Methods 2003; 274: 245–256.
Leclere PG, Panjwani A, Docherty R, Berry M, Pizzey J, Tonge DA . Effective gene delivery to adult neurons by a modified form of electroporation. J Neurosci Methods 2005; 142: 137–143.
Aluigi M, Fogli M, Curti A, Isidori A, Gruppioni E, Chiodoni C et al. Nucleofection is an efficient nonviral transfection technique for human bone marrow-derived mesenchymal stem cells. Stem Cells 2006; 24: 454–461.
Dubois SG, Floyd EZ, Zvonic S, Kilroy G, Wu X, Carling S et al. Isolation of human adipose-derived stem cells from biopsies and liposuction specimens. Methods Mol Biol 2008; 449: 69–79.
Li S . Optimizing electrotransfection of mammalian cells in vitro. In: Freidmann T (ed). Gene Transfer: Delivery and Expression of DNA and RNA: A Laboratory Manual. Cold Spring Harbor Laboratory Press, 2006, pp 419–425.
Prabha S, Zhou WZ, Panyam J, Labhasetwar V . Size-dependency of nanoparticle-mediated gene transfection: studies with fractionated nanoparticles. Int J Pharm 2002; 244: 105–115.
Moore NM, Barbour TR, Sakiyama-Elbert SE . Synthesis and characterization of four-arm poly(ethylene glycol)-based gene delivery vehicles coupled to integrin and DNA-binding peptides. Mol Pharm 2008; 5: 140–150.
Saxena A, Mozumdar S, Johri AK . Ultra-low sized cross-linked polyvinylpyrrolidone nanoparticles as non-viral vectors for in vivo gene delivery. Biomaterials 2006; 27: 5596–5602.
Kabanov AV, Batrakova EV, Alakhov VY . Pluronic block copolymers as novel polymer therapeutics for drug and gene delivery. J Control Release 2002; 82: 189–212.
Hartikka J, Sukhu L, Buchner C, Hazard D, Bozoukova V, Margalith M et al. Electroporation-facilitated delivery of plasmid DNA in skeletal muscle: plasmid dependence of muscle damage and effect of poloxamer 188. Mol Ther 2001; 4: 407–415.
Kabanov AV, Lemieux P, Vinogradov S, Alakhov V . Pluronic block copolymers: novel functional molecules for gene therapy. Adv Drug Deliv Rev 2002; 54: 223–233.
Stroh T, Erben U, Kuhl AA, Zeitz M, Siegmund B . Combined pulse electroporation—a novel strategy for highly efficient transfection of human and mouse cells. PLoS One 2010; 5: e9488.
Gresch O, Engel FB, Nesic D, Tran TT, England HM, Hickman ES et al. New non-viral method for gene transfer into primary cells. Methods 2004; 33: 151–163.
Zaharoff DA, Henshaw JW, Mossop B, Yuan F . Mechanistic analysis of electroporation-induced cellular uptake of macromolecules. Exp Biol Med (Maywood) 2008; 233: 94–105.
Kucerova L, Altanerova V, Matuskova M, Tyciakova S, Altaner C . Adipose tissue-derived human mesenchymal stem cells mediated prodrug cancer gene therapy. Cancer Res 2007; 67: 6304–6313.
Acknowledgements
This study was supported by National Institutes of Health grant RO1CA120895 and NIH/NIBIB grant R21EB007208. Authors also are thankful to the assistance in cell culture from Jiemiao Hu and in plasmid DNA preparation by Xueqing Xia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Cancer Gene Therapy website
Supplementary information
Rights and permissions
About this article
Cite this article
Flanagan, M., Gimble, J., Yu, G. et al. Competitive electroporation formulation for cell therapy. Cancer Gene Ther 18, 579–586 (2011). https://doi.org/10.1038/cgt.2011.27
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cgt.2011.27
Keywords
This article is cited by
-
Competitive DNA transfection formulation via electroporation for human adipose stem cells and mesenchymal stem cells
Biological Procedures Online (2012)