Abstract
Prader–Willi syndrome (PWS) is a highly variable genetic disorder affecting multiple body systems whose most consistent major manifestations include hypotonia with poor suck and poor weight gain in infancy; mild mental retardation, hypogonadism, growth hormone insufficiency causing short stature for the family, early childhood-onset hyperphagia and obesity, characteristic appearance, and behavioral and sometimes psychiatric disturbance. Many more minor characteristics can be helpful in diagnosis and important in management. PWS is an example of a genetic condition involving genomic imprinting. It can occur by three main mechanisms, which lead to absence of expression of paternally inherited genes in the 15q11.2–q13 region: paternal microdeletion, maternal uniparental disomy, and imprinting defect.
Similar content being viewed by others
In brief
-
PWS is a common and complex disorder affecting multiple systems
-
Early diagnosis is important to effective long-term management
-
Hypotonia, beginning prenatally, causes poor feeding and development in the perinatal period and infancy
-
If untreated obesity typically begins after 1–2 years of age and is later exacerbated by hyperphagia with lack of satiety
-
The major cause of morbidity and mortality is morbid obesity
-
Obesity can be controlled externally by diet restrictions and behavior modification
-
Parental education about appropriate parenting techniques (structure and consistency) for the behavioral and eating issues of PWS correlates with prognosis
-
Growth hormone treatment improves growth, physical phenotype and body composition
-
Clinical diagnostic criteria have been developed, but must be confirmed with genetic testing because of clinically overlapping disorders, particularly as an infant
-
Over 99% can be diagnosed with a simple molecular test, DNA methylation analysis
-
Three major genetic causes exist: 5–7 Mb deletion of the paternally inherited chromosomal 15q11.2–q13 region, maternal uniparental disomy 15, and a defect in the imprinting process in the 15q11.2–q13 region on the paternally inherited chromosome. Occasionally, deletion results from chromosomal translocation
-
A number of imprinted genes have been mapped to the PWS region of 15q11.2–q13, but their role in most aspects of the phenotype is not well understood.
Introduction
Prader–Willi syndrome (PWS; OMIM 176270) is a relatively common (prevalence 1/15 000–1/30 000) generally sporadic disorder with a recognizable pattern of dysmorphic features and major neurologic, cognitive, endocrine and behavioral/psychiatric disturbances. PWS was the first recognized human disorder related to genomic imprinting and the first shown to be caused by uniparental disomy. It results from failure of expression of paternally inherited genes in the PWS region of chromosome 15. Major characteristics include infantile lethargy and hypotonia causing poor feeding and failure to thrive, developmental and intellectual disability, hypogonadism (small external genitalia and pubertal insufficiency), hyperphagia leading to morbid obesity if uncontrolled, short stature, characteristic facial appearance and body habitus, and a typical behavioral phenotype that includes temper tantrums and compulsive traits. Management is symptomatic and supportive, emphasizing control of food intake, hormone replacement therapies, special education and sheltered employment, and behavior management.
Clinical overview
Hypotonia and abnormal neurologic function
Hypotonia is prenatal in onset, and is usually manifested as decreased fetal movement, abnormal fetal position at delivery, and increased need for assisted delivery or cesarean section. In infancy (Figure 1), there is decreased movement and lethargy with decreased spontaneous arousal, weak cry, and poor reflexes, including a poor suck that leads to early feeding difficulties and poor weight gain. Deep tendon reflexes are often spared. Assisted feeding through a feeding tube and/or special nipples with increased feeding times are invariably necessary. The hypotonia is central in origin. Mild-to-moderate hypotonia persists throughout life. Hypotonia is so characteristic that all newborns with unexplained persistent hypotonia should be tested for PWS.1, 2
Hypogonadism
In both sexes, hypogonadism manifests as genital hypoplasia throughout life, incomplete pubertal development, and infertility in the vast majority.3 In males, the penis may be small, but most characteristic is a hypoplastic scrotum that is small, poorly rugated, and poorly pigmented (Figure 1). Unilateral or bilateral cryptorchidism is present in 80–90%. In females, the labia (majora and minora) and clitoris are generally hypoplastic. Precocious adrenarche occurs in both sexes in up to about 20%. Delayed and incomplete pubertal development is characteristic. Menarche may occur as late as the 30 s, and usually there is amenorrhea or oligomenorrhea. In males, there is poor voice change and scanty beard and body hair. In both sexes, little or nothing is known about sexual activity, which is believed to be deficient, and most affected individuals are presumed to be infertile. Although very rare, several instances of reproduction have been reported, all in females. The hypogonadism is of hypothalamic origin, and generally there is hypogonadotropism with decreased testosterone or estrogen and decreased FSH and LH in both sexes.
Developmental and cognitive delays
Gross motor and language milestones are delayed. Early milestones are reached on average at double the normal age (eg, sitting at 12 months, walking at 24 months, and words at 2 years). Cognitive disability is evident by school age. Most people with PWS are mildly mentally retarded (mean IQ: 60–70 s), with approximately 40% having borderline mental retardation or low-normal intelligence and approximately 20% having moderate retardation.4, 5, 6 Regardless of measured IQ, most people with PWS have multiple severe learning disabilities and poor academic performance for their mental abilities. Articulation is poor.7 Most adults require sheltered residential and employment settings because of a combination of cognitive, behavioral and food-seeking characteristics.
Hyperphagia and obesity
Obesity typically begins between 1–4 years. In later childhood a seemingly insatiable appetite (hyperphagia) begins. The hyperphagia is hypothalamic in origin, resulting in lack of sense of satiety.8, 9, 10, 11 Food seeking is common (hording, foraging for food, eating of unappealing food items, and stealing of food or money to buy food). If intake is not controlled externally, central obesity results from these behaviors combined with a low metabolic rate and decreased activity level (that result in a decreased total caloric requirement) (Figure 2). Complications of obesity are the major causes of morbidity and mortality: cardiorespiratory insufficiency, obstructive sleep apnea, thrombophlebitis, and chronic leg edema. Up to 25% of obese adults have type II diabetes mellitus (NIDDM),12 with mean age of onset of 20 years.
Short stature
Short stature is almost always present by the second decade in the absence of growth hormone replacement, and a deficient pubertal growth spurt results in an average adult height of 155 cm for males and 148 cm for females.13 Data from multiple studies involving over 400 children and adults with PWS document reduced growth hormone secretion (see also Management section).
Behavioral and psychiatric disturbances
A characteristic behavioral pattern begins in early childhood in 70–90% of affected individuals. It is typified by temper tantrums, stubbornness, controlling and manipulative behavior, compulsive-like behaviors (repeated organizing, writing, collecting, need to finish one thing before moving to the next), and difficulty with change in routine.4, 14, 15, 16, 17 Many of the behavioral characteristics are suggestive of autism spectrum disorder, which has been diagnosed in up to 25% of the individuals.18 Attention deficit/hyperactivity symptoms and insistence on sameness are common and of early onset.19 The severity of behavioral problems increases with age and body mass index,20 and then diminishes in older adults.21 Psychosis is evident by young adulthood in at least 5–10% of the individuals.17, 22, 23
Other characteristics
A number of less consistent features are nonetheless common and/or characteristic, and may be important for diagnosis or management (Figures 2 and 3; Table 1)
Sleep abnormalities
Individuals with PWS have sleep-disordered breathing, including central and obstructive sleep apnea, abnormal arousal, abnormal circadian rhythm in REM sleep, reduced REM latency, and abnormal response to hypercapnia26, 27 as well as excessive daytime sleepiness. Obesity can worsen the sleep disorder.
Mortality
Several reports of deaths in PWS28, 29 and surveys within large cohorts30, 31 found that obesity-related cardiovascular and respiratory disorders were the most frequent causes of death in both children and adults. Based on a population study, the death rate has been estimated at 3% per year.12 Another large study suggested a 6-fold relative risk of death in PWS versus other developmentally disabled individuals.30 Specific concerns have been raised about eating-related fatalities, including choking on gorged food32 and gastric necrosis and rupture following binging, particularly in slim but previously obese individuals.33 Recently, it was found that central adrenal insufficiency (CAI) was present in 60% of tested individuals with PWS and the authors suggest that CAI during times of stress could explain many of the sudden and unexplained deaths in PWS.34
Diagnostic approaches
Clinical diagnosis
Although consensus clinical diagnostic criteria have been published35 and validated, they were published before comprehensive laboratory testing for PWS was available. Diagnosis should not rest on clinical grounds alone. Revised criteria, designed to trigger diagnostic testing, were published based on the frequency of criteria in molecularly proven individuals2 (Table 2).
Diagnostic testing
Although the DNA sequence of imprinted maternally and paternally inherited alleles is the same, multiple epigenetic factors (such as DNA methylation) ultimately will determine if the allele is expressed or repressed. The differential DNA methylation of certain maternal and paternal alleles in the 15q11.2–q13 region provides a tool for assessing paternal-only, maternal-only and normal (biparental) inheritance.
DNA methylation analysis is the only technique that will diagnose PWS correctly in all three molecular classes36 as well as differentiate PWS from the clinically distinct Angelman syndrome in deletion cases.37 It is frequently the first genetic test used for PWS. The most robust and most widely used DNA methylation test targets the 5′ end of the SNURF-SNRPN (typically referred to as SNRPN) locus.36, 38 The promoter region of SNRPN is unmethylated on the paternally expressed allele and methylated on the maternally repressed allele. Normal individuals have both a methylated and an unmethylated allele, whereas individuals with PWS have only the maternally methylated allele.
DNA methylation cannot distinguish the molecular class (Figure 4). Deletion of the paternally contributed 15q11.2–q1339, 40 occurs in about 70% and is diagnosed typically using the SNRPN fluorescence in situ hybridization (FISH) probe.41 Chromosome analysis should be included in testing for a deletion, as occasionally the deletion is a result of a chromosomal translocation. Maternal uniparental disomy (UPD) 1542 (ie, two chromosomes 15 from the mother and none from the father) occurs in about 20–30% of individuals. This class is diagnosed using DNA polymorphism analysis on the proband's and parents' DNA. The third molecular class is the imprinting defects. These disrupt the imprinting process on the paternally inherited chromosome 15. They account for about 2–5% of individuals. Most imprinting defects are epigenetic (epimutations) and demonstrate a maternal-only DNA methylation pattern despite the presence of both the parental alleles (biparental). DNA sequence changes are not found in these epimutations and they are thought to be random stochastic errors occurring in spermatogenesis of the fathers.43 By contrast, about 15% of individuals with an imprinting defect are found to have a very small deletion in the PWS imprinting center region located at the 5′ end of the SNRPN gene. Tests for the specific mechanism causing PWS are useful for genetic counselling purposes (see Genetic counselling section).
Molecular classes of PWS and their frequencies. Deletion 15q11.2–q13, maternal uniparental disomy (UPD), imprinting defect (ID) and single gene mutation. The parent of origin of each chromosome is indicated with M (maternal) or P (paternal). Note that there is biparental contribution of the two chromosomes 15 in the case of ID, but the paternal (P) contribution in 15q11.2–q13 has maternal (M) epigenetic markings (eg, DNA methylation) and behaves accordingly.
Differential diagnosis
Several disorders that can strongly resemble PWS should be considered during the diagnostic process. Infantile hypotonia has a very long list of causes, central and peripheral, syndromic and isolated. Angelman syndrome (AS) must be considered if the diagnosis was made in an infant by FISH, because AS also presents with hypotonia and feeding difficulties. AS is also frequently caused by a 15q11.2–q13 deletion (70%), but on the maternal 15. Therefore all infants less than 2 years of age with a deletion should have DNA methylation testing to distinguish AS and PWS. A subset of individuals with Fragile X syndrome can become hyperphagic and obese. A number of other conditions associate obesity and developmental disability, including UPD 14, Cohen syndrome, Bardet–Biedl syndrome, Alstrom syndrome, duplications of 3p25.3–p26.2 and of Xq27.2-ter and deletions 1p36, 6q16.2, and 10q26.
Molecular and genetic basis of the disease
A genomic, imprinted, contiguous gene disorder
PWS is an example of a ‘genomic disorder’, as it results from altered genomic structure (an epigenetic phenomenon) and not a specific DNA sequence change. The various genomic changes causing PWS all lead to loss of expression of the paternally expressed genes on chromosome 15q11.2–q13 through loss or failure of expression because the maternal contribution has been programmed by epigenetic factors (eg, DNA methylation) to be silenced.41
The structure of the PWS region
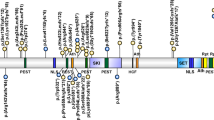
Most cases of PWS result from a deletion of 5–7 Mb in 15q11.2–q13. This region is highly complex and contains a number of imprinted and non-imprinted genes (Figure 5). The vast majority of individuals with deletions have one of two common proximal breakpoints (BP1 and BP2) and a common distal breakpoint (BP3).44, 45, 46, 47 This is on account of the presence of low copy repeat sequences flanking the deleted region,45 which may result in aberrant recombination of the segment during meiosis.
Summary of the genetic and expression map of chromosomal region 15q11.2–q13. The position of genes and genetic markers (circles) are shown. In the PWS region (shown in blue), there are six paternal-only (PWS region) expressed unique copy genes (MKRN3, MAGEL2, NECDIN, C15ORF2 and SNURF- SNRPN and a family of five paternal-only expressed snoRNA genes). Only UBE3A and ATP10A (shown in red), related to Angelman syndrome (AS), have maternal-only expression in mouse and humans, and this imprinted expression is limited to certain tissue-specific regions (ie, brain). The bipartite imprinting center (IC) lies proximal to SNURF-SNRPN and within the 3 Mb PWS/AS imprinted region. The cluster of GABA receptor genes (GABRB3, GABRA5 and GABRG3), OCA2 (type II albinism) and HERC2 are not imprinted and have biparental expression (shown in black). The jagged vertical lines denote the common 5–7 Mb AS and PWS deletion breakpoints, which lie within the segmental duplications associated with BP1, BP2 and BP3.45 There are two common proximal breakpoints and one distal common breakpoint.44 In between BP1 and BP2 lie four additional, non-imprinted genes GCP5 (TUBGCP5), CYFIP1, NIPA2 and NIPA1.46 Type 1 deletions extend from BP1 to BP3 and type 2 deletions extend from BP2 to BP3. Note that there are more copies of the HBII-85 and HBII-52 genes than are shown.
The genes related to PWS
The 15q11.2–q13 region can be roughly divided into four distinct regions (Figure 5): (1) a proximal non-imprinted region between BP1 and BP2 containing four biparentally expressed genes; (2) a ‘PWS paternal-only expressed region’ containing five protein coding genes (MKRN3, MAGEL2, NECDIN, and the bicistronic SNURF-SNRPN), a cluster of five repetitious snoRNA genes (HBII-436, HBII-13, HBII-438, HBII-85 and HBII-52) and several antisense transcripts (including the antisense transcript to UBE3A); (3) an ‘AS region’ containing the preferentially maternally expressed genes UBE3A and ATP10A and (4) a distal non-imprinted region containing a cluster of three GABA receptor genes, the gene for oculocutaneous albinism type 2 (OCA2) and the HERC2 gene. The exact function of each of these genes in the PWS phenotype is still being elucidated. The various created gene knock out mouse models will be useful in uncovering these contributions. Although no single gene alteration has been found that explains all the features of PWS, several unique translocation and deletion patients have narrowed a ‘key’ region to explain much of the PWS phenotype to the HBII-85 snoRNA gene (reviewed in Sahoo et al).48
Genotype–phenotype correlations
Individuals with UPD are less likely to have the hypopigmentation,49 typical facial appearance,50, 51 or skill with jigsaw puzzles;52 they also have a somewhat higher verbal IQ and milder behavioral problems.53, 54 However, psychosis55 and autism spectrum disorders56, 57 occur with significantly greater frequency among those with uniparental disomy. A recent report suggests that individuals with type 1 deletions (BP1 to BP3) had more compulsions and poorer adaptive behavior, intellectual ability, and academic achievement than those with type 2 deletions (BP2 to BP3).46 However, two other studies found much more subtle differences.58, 59
Management
Management of PWS is focused on anticipatory guidance and addressing the consequences of the syndrome, and is very age-dependent. Detailed discussions can be found elsewhere.60, 61 Early diagnosis, preferably in the nursery, offers the opportunity to greatly improve health and quality of life in people with PWS and their families.
Initial evaluation
Please see Table 3 for recommended evaluations at the time of initial diagnosis. Genetic counselling should also take place at the time of diagnosis, as should referral to support organizations.
Treatment and care
Hypotonia
In infancy, the major focus should be on assuring adequate nutrition and growth. Compensation for the poor suck almost always requires gavage feeding or use of special nipples (eg, enlarged nipple holes or those designed for cleft palate). As infants rarely wake to feed, a regular feeding schedule should be established. Caloric intake should be assessed, and height, weight and head circumference plotted on growth charts monthly for the first 6 months and quarterly for 12–24 months. Feeding problems are transient, so a gastrostomy tube is rarely necessary. Fats should not be restricted as they are essential for brain development.
Early infant stimulation programs are strongly recommended to assure adequate interactions and optimize strength and milestone achievement. Encouragement of physical activity is important for strength and agility at all ages.
Scoliosis can occur at any time throughout childhood and is treated in a standard manner.
Obesity predisposition
Nutritional counselling for good long-term weight management should begin in early infancy to prevent the inappropriate weight gain that would otherwise typically begin between 12–36 months of age. Caloric requirement is low on account of decreased calorie utilization, often as low as 60% of that for unaffected children of the same body weight62 so that care should be taken to not overfeed the infant with PWS. It is important to monitor growth and record measurements on a standard growth curve regularly (monthly in early infancy, every 2–3 months from 2 to 6 years, and at least two times yearly thereafter throughout childhood) so that excess weight gain can be identified and dealt with early. At least twice yearly weights in adulthood, with comparison to prior weights, are also important to monitor for weight gain. Vitamin and mineral intake (especially calcium and vitamin D) should be monitored, and supplements given as needed.
Once hyperphagia begins, the feeling of hunger is typically never completely relieved, even after a large meal. No known pharmacologic agent is effective. This lack of satiety should be considered at school, work and home, and all caretakers and supervisors need to understand these factors. Consistent limit setting and close supervision at all ages, including locking of cabinets and refrigerator at home and avoidance of work environments with available food, decreases anxiety and conflict. It is imperative to begin good meal management and education at an early age. This includes sticking to a strict schedule for meals and snacks, and limiting portion sizes. Exercise is also a very important factor in weight maintenance, and early establishment of a routine of regular daily physical activity (at least 30 min) is strongly recommended. More and more individuals with PWS are now growing up having avoided the obesity that used to be so typical of this syndrome (Figure 6).
Normalization of the body and facial phenotype of PWS following treatment with growth hormone from infancy. (a) A 7-year-old unaffected sibling on the left and his 8-year-old brother with PWS on the right. Note lighter pigmentation in affected brother who has a typical deletion, and scar from the gastrostomy tube used in infancy. The unaffected brother has 24% body fat and the brother with PWS has 20% body fat. (b) A 2-year-old boy. (c) A 4-year-old unrelated boy.
Growth deficiency
The benefits of growth hormone therapy in infants, children and adults with PWS have been well-demonstrated in multiple well-designed and well-controlled studies.63, 64, 65, 66, 67 Growth hormone replacement therapy improves linear growth velocity and ultimate height, body composition (increased lean body mass, decreased fat mass), muscle function, and level of activity. There is evidence of improvement in the respiratory drive and function. Growth hormone helps maintain muscle bulk and body composition in adults. When treatment occurs from infancy, facial appearance and body habitus normalize in conjunction with good dietary management (Figure 6). Rare side effects of human growth hormone therapy include pedal edema, hastening of scoliosis, slipped capital femoral epiphysis and risk of pseudotumor cerebri. Premature adrenarche has been reported to occur more commonly.65 Because of concerns about unexpected death on account of respiratory obstruction early in growth hormone treatment, sleep polysomnography and assessment for enlarged tonsils and adenoids are recommended before initiating growth hormone treatment and 6–8 weeks after starting.68 However, there is controversy among experts about a direct role of growth hormone in these unexpected deaths.31, 63, 68 In addition, thyroid function should be assessed before growth hormone therapy, as 15% of patients have hypothyroidism. Serum IGF-1 levels, growth velocity and head circumference should be monitored during treatment and kept in the normal range.68
Hypogonadism
Cryptorchidism in males is common and often requires surgical intervention. A trial of chorionic gonadotropin will enlarge the scrotum so it can better contain the testes post-surgically, and might circumvent the need for surgery. Pubertal deficiency can be treated with replacement of sex hormones, which produces adequate secondary sexual characteristics. Concerns about testosterone replacement possibly causing behavior problems in males have been largely alleviated by daily administration through the patch or gel versus previously used monthly intramuscular depot injections. In females there are concerns about hygiene issues with monthly menstruation and the increased risk of strokes with estrogen replacement. Few well-designed studies of sex hormone replacement are published. The high frequency of osteopenia/osteoporosis in PWS argues in favor of sex hormone replacement. Although sex drive appears decreased in PWS, sex education should not be neglected because of the risk of sexually transmitted diseases and the rare pregnancy in females.
Developmental disability
Children with PWS should have early intervention (including physical, occupational, and speech therapies) and individualized appropriate education. This ideally includes social skills training. Optimally, a one-to-one aide in the classroom will help the child focus on learning and diminish behavioral disturbances. Adults require sheltered employment and housing.
Behavior and psychiatric problems
Behavioral problems should be detected early and treated appropriately with parental education/training (including consistent limit setting) and, if needed, consideration of counselling and/or psychotropic medication. Serotonin agonists have been the most successful in reducing temper outbursts and improving compulsivity.69, 70, 71 Families should be made aware of the signs of psychosis and urged to have psychiatric assessment early if a thought disorder is apparent. It is particularly important to be aware that psychosis develops in a substantial number of adults with UPD or imprinting defects.22, 23 Psychosis is treated in a standard manner.
Genetic counselling
Recurrence risk is dependent on the genetic mechanism causing PWS in the individual. Deletion 15q11.2–q13 is sporadic (recurrence <1%) except in the rare cases where a chromosomal rearrangement (translocation or inversion) is present in the father. Fathers of children with deletion should be offered chromosomal and FISH analysis of the 15q11.2–q13 region as the recurrence risk is significantly increased in these cases. Maternal UPD 15 is typically de novo (recurrence <1%) except if a Robertsonian translocation is present in either parent, so a chromosomal analysis is indicated. If this is normal, then the father of the child should be offered a chromosomal analysis to ensure that he does not have a Robertsonian translocation. A proportion of those with an imprinting defect have a microdeletion in the Imprinting Center; this can be familial and has a 50% recurrence risk when it is. However, the greater proportion of those with an imprinting defect have an epigenetic mutation and the recurrence risk is <1% for this group.
PWS cannot be identified clinically before birth. FISH, DNA polymorphism studies for UPD, and DNA methylation analysis have all been validated for prenatal diagnosis in amniocytes or chorionic villi, and all three are available clinically. Only DNA methylation analysis will identify the imprinting defects.72
Conclusion
Growth hormone replacement in PWS has resulted in dramatic benefits to the phenotype, health and self-image of those treated. But much is yet to be learned about this complex disorder. Clinically, the most urgent need is arguably to identify the cause of hyperphagia in hopes of finding pharmacologic agents that can diminish its impact. Would the effect of such a drug be to improve behavior and cognitive ability as well as health? If obesity could be more easily avoided, the major cause of morbidity and mortality would be eliminated and significantly improved health and quality of life could be expected.
It has been 27 years since the genetic region responsible for PWS was first identified; yet we still do not know the precise gene(s) responsible for the phenotype. Recent data suggests a key role for the HBII-85 snoRNA gene,48, 73 and the near future should uncover its gene targets and hopefully lead to treatments. Identification of the responsible gene(s) may also be extremely instructive concerning causes of obesity, hypogonadism, other hypothalamic deficiencies, psychosis, and autism in the general population. PWS has already taught us much about imprinting, and may have much more to teach us.
References
Miller SP, Riley P, Shevell MI : The neonatal presentation of Prader-Willi syndrome revisited. J Pediatr 1999; 134: 226–228.
Gunay-Aygun M, Schwartz S, O'Riordan MA, Cassidy SB : The changing purpose of Prader-Willi syndrome clinical diagnostic criteria and proposed revised criteria. Pediatrics 2001; 108: E92.
Crinó A, Schiaffini R, Ciampalini P et al: Hypogonadism and pubertal development in Prader-Willi syndrome. Eur J Pediatr 2003; 162: 327–333.
Dykens EM, Hodapp RM, Walsh K, Nash L : Profiles, correlates and trajectories of intelligence in individuals with Prader-Willi syndrome. J Am Acad Child Adolesc Psychiatry 1992; 31: 1125–1130.
Curfs LM, Fryns JP : Prader-Willi syndrome: a review with special attention to the cognitive and behavioral profile. Birth Defects Orig Artic Ser 1992; 28: 99–104.
Malich S, Largo RH, Schinzel A, Molinari L, Eiholzer U : Phenotypic heterogeneity of growth and psychometric intelligence in Prader-Willi syndrome: variable expression of a contiguous gene syndrome or parent-child resemblance? Am J Med Genet 2000; 91: 298–304.
Lewis BA, Freebairn L, Heeger S, Cassidy SB : Speech and language skills of individuals with Prader-Willi syndrome. Am J Speech Lang Pathol 2002; 11: 1–10.
Zipf WB, Berntson GG : Characteristics of abnormal food-intake patterns in children with Prader-Willi syndrome and study of effects of naloxone. Am J Clin Nutr 1987; 46: 277–281.
Holland AJ, Treasure J, Coskeran P, Dallow J, Milton N, Hillhouse E : Measurement of excessive appetite and metabolic changes in Prader-Willi syndrome. Int J Obes.Relat Metab Disord 1993; 17: 527–532.
Holland AJ, Treasure J, Coskeran P, Dallow J : Characteristics of the eating disorder in Prader-Willi syndrome: implications for treatment. J Intellect Disabil Res 1995; 39: 373–381.
Swaab DF, Purba JS, Hofman MA : Alterations in the hypothalamic paraventricular nucleus and its oxytocin neurons (putative satiety cells) in Prader-Willi syndrome: a study of five cases. J Clin Endocrinol Metab 1995; 80: 573–579.
Butler JV, Whittington JE, Holland AJ, Boer H, Clarke D, Webb T : Prevalence of, and risk factors for, physical ill-health in people with Prader-Willi syndrome: a population-based study. Dev Med Child Neurol 2002; 44: 248–255.
Butler MG, Meaney FJ : Standards for selected anthropometric measurements in Prader-Willi syndrome. Pediatr 1991; 88: 853–860.
Dykens EM, Cassidy SB : Correlates of maladaptive behavior in children and adults with Prader- Willi syndrome. Am J Med Genet 1995; 60: 546–549.
Clarke DJ, Boer H, Chung MC, Sturmey P, Webb T : Maladaptive behaviour in Prader-Willi syndrome in adult life. J Intellect Disabil Res 1996; 40: 159–165.
State MW, Dykens EM, Rosner B, Martin A, King BH : Obsessive-compulsive symptoms in Prader-Willi and ‘Prader-Willi-Like’ patients. J Am Acad Child Adolesc Psychiatry 1999; 38: 329–334.
Clarke DJ, Boer H, Whittington J, Holland A, Butler J, Webb T : Prader-Willi syndrome, compulsive and ritualistic behaviours: the first population-based survey. Br J Psychiatry 2002; 180: 358–362.
Veltman MW, Craig EE, Bolton PF : Autism spectrum disorders in Prader-Willi and Angelman syndromes: a systematic review. Psychiatr Genet 2005; 15: 243–254.
Wigren M, Hansen S : ADHD symptoms and insistence on sameness in Prader-Willi syndrome. J Intellect Disabil Res 2005; 49: 449–456.
Steinhausen HC, Eiholzer U, Hauffa BP, Malin Z : Behavioural and emotional disturbances in people with Prader-Willi syndrome. J Intellect Disabil Res 2004; 48: 47–52.
Dykens EM : Maladaptive and compulsive behavior in Prader-Willi syndrome: new insights from older adults. Am J Ment Retard 2004; 109: 142–153.
Boer H, Holland A, Whittington J, Butler J, Webb T, Clarke D : Psychotic illness in people with Prader-Willi syndrome due to chromosome 15 maternal uniparental disomy. Lancet 2002; 359: 135–136.
Vogels A, De Hert M, Descheemaeker MJ et al: Psychotic disorders in Prader-Willi syndrome. Am J Med Genet A 2004; 127: 238–243.
West LA, Ballock RT : High incidence of hip dysplasia but not slipped capital femoral epiphysis in patients with Prader-Willi syndrome. J Pediatr Orthop 2004; 24: 565–567.
Hart PS : Salivary abnormalities in Prader-Willi syndrome. Ann N Y Acad Sci. 1998; 842: 125–131.
Nixon GM, Brouillette RT : Sleep and breathing in Prader-Willi syndrome. Pediatr Pulmonol 2002; 34: 209–217.
Yee BJ, Buchanan PR, Mahadev S et al: Assessment of sleep and breathing in adults with Prader-Willi syndrome: a case control series. J Clin Sleep Med 2007; 3: 713–718.
Schrander-Stumpel CT, Curfs LM, Sastrowijoto P, Cassidy SB, Schrander JJ, Fryns JP : Prader-Willi syndrome: causes of death in an international series of 27 cases. Am J Med Genet A 2004; 124: 333–338.
Stevenson DA, Anaya TM, Clayton-Smith J et al: Unexpected death and critical illness in Prader-Willi syndrome: report of ten individuals. Am J Med Genet A 2004; 124: 158–164.
Einfeld SL, Kavanagh SJ, Smith A, Evans EJ, Tonge BJ, Taffe J : Mortality in Prader-Willi syndrome. Am J Ment Retard 2006; 111: 193–198.
Grugni G, Crinò A, Bosio L et al: The Italian National Survey for Prader-Willi syndrome: An epidemiologic study. Am J Med Genet A 2008; 146: 861–872 e-pub ahead of print 17 January.
Stevenson DA, Heinemann J, Angulo M et al: Deaths due to choking in Prader-Willi syndrome. Am J Med Genet A 2007; 143: 484–487.
Stevenson DA, Heinemann J, Angulo M et al: Gastric rupture and necrosis in Prader-Willi syndrome. J Pediatr Gastroenterol Nutr 2007; 45: 272–274.
de Lind van Wijngaarden RF, Otten BJ, Festen DA et al: High prevalence of central adrenal insufficiency in patients with Prader-Willi syndrome. J Clin Endocrinol Metab 2008; 93: 1649–1654. e-pub ahead of print 26 February 2008.
Holm VA, Cassidy SB, Butler MG et al: Prader-Willi syndrome: consensus diagnostic criteria. Pediatrics 1993; 91: 398–402.
Glenn CC, Saitoh S, Jong MT et al: Gene structure, DNA methylation, and imprinted expression of the human SNRPN gene. Am J Hum Genet 1996; 58: 335–346.
Driscoll DJ, Waters MF, Williams CA et al: A DNA methylation imprint, determined by the sex of the parent, distinguishes the Angelman and Prader-Willi syndromes. Genomics 1992; 13: 917–924.
Kubota T, Das S, Christian SL, Baylin SB, Herman JG, Ledbetter DH : Methylation-specific PCR simplifies imprinting analysis. Nat Genet 1997; 16: 16–17.
Ledbetter DH, Riccardi VM, Airhart SD, Strobel RJ, Keenan BS, Crawford JD : Deletions of chromosome 15 as a cause of the Prader-Willi syndrome. N Engl J Med 1981; 304: 325–329.
Butler MG, Palmer CG : Parental origin of chromosome 15 deletion in Prader-Willi syndrome. Lancet 1983; 1: 1285–1286.
Glenn CC, Driscoll DJ, Yang TP, Nicholls RD : Genomic imprinting: potential function and mechanisms revealed by the Prader-Willi and Angelman syndromes. Mol Hum Reprod 1997; 3: 321–332.
Nicholls RD, Knoll JH, Butler MG, Karam S, Lalande M : Genetic imprinting suggested by maternal heterodisomy in nondeletion Prader-Willi syndrome. Nature 1989; 342: 281–285.
Buiting K, Gross S, Lich C, Gillessen-Kaesbach G, el-Maarri O, Horsthemke B : Epimutations in Prader-Willi and Angelman syndromes: a molecular study of 136 patients with an imprinting defect. Am J Hum Genet 2003; 72: 571–577.
Christian SL, Robinson WP, Huang B et al: Molecular characterization of two proximal deletion breakpoint regions in both Prader-Willi and Angelman syndrome patients. Am J Hum Genet 1995; 57: 40–48.
Amos-Landgraf JM, Ji Y, Gottlieb W et al: Chromosome breakage in the Prader-Willi and Angelman syndromes involves recombination between large, transcribed repeats at proximal and distal breakpoints. Am J Hum Genet 1999; 65: 370–386.
Butler MG, Bittel DC, Kibiryeva N, Talebizadeh Z, Thompson T : Behavioral differences among subjects with Prader-Willi syndrome and type I or type II deletion and maternal disomy. Pediatrics 2004; 113: 565–573.
Robinson WP, Dutly F, Nicholls RD et al: The mechanisms involved in formation of deletions and duplications of 15q11-q13. J Med Genet 1998; 35: 130–136.
Sahoo T, del Gaudio D, German JR et al: Prader-Willi phenotype caused by paternal deficiency for the HBII-85 C/D box small nucleolar RNA cluster. Nat Genet 2008; 40: 719–721.
Butler MG : Hypopigmentation: a common feature of Prader-Labhart-Willi syndrome. Am J Hum Genet 1989; 45: 140–146.
Gillessen-Kaesbach G, Robinson W, Lohmann D, Kaya-Westerloh S, Passarge E, Horsthemke B : Genotype-phenotype correlation in a series of 167 deletion and non-deletion patients with Prader-Willi syndrome. Hum Genet 1995; 96: 638–643.
Cassidy SB, Forsythe M, Heeger S, Nicholls R, Schork N, Schwartz S : Comparison of phenotypic features between patients with Prader-Willi syndrome due to deletion 15q and uniparental disomy 15. Am J Med Genet 1997; 68: 433–440.
Dykens EM : Are jigsaw puzzle skills ‘spared’ in persons with Prader-Willi syndrome? J Child Psychol Psychiatry 2002; 43: 343–352.
Dykens EM, Cassidy SB, King BH : Maladaptive behavior differences in Prader-Willi syndrome due to paternal deletion versus maternal uniparental disomy. Am J Ment Retard 1999; 104: 67–77.
Roof E, Stone W, MacLean W, Feurer ID, Thompson T, Butler MG : Intellectual characteristics of Prader-Willi syndrome: comparison of genetic subtypes. J Intellect Disabil Res 2000; 44: 25–30.
Holland AJ, Whittington JE, Butler J, Webb T, Boer H, Clarke D : Behavioural phenotypes associated with specific genetic disorders: evidence from a population-based study of people with Prader-Willi syndrome. Psychol Med 2003; 33: 141–153.
Veltman MW, Thompson RJ, Roberts SE, Thomas NS, Whittington J, Bolton PF : Prader-Willi syndrome – a study comparing deletion and uniparental disomy cases with reference to autism spectrum disorders. Eur Child Adolesc Psychiatry 2004; 13: 42–50.
Whittington J, Holland A, Webb T, Butler J, Clarke D, Boer H : Cognitive abilities and genotype in a population-based sample of people with Prader-Willi syndrome. J Intellect Disabil Res 2004; 48: 172–187.
Milner KM, Craig EE, Thompson RJ et al: Prader-Willi syndrome: intellectual abilities and behavioural features by genetic subtype. J Child Psychol Psychiatry 2005; 46: 1089–1096.
Varela MC, Kok F, Setian N, Kim CA, Koiffmann CP : Impact of molecular mechanisms, including deletion size, on Prader-Willi syndrome phenotype: study of 75 patients. Clin Genet 2005; 67: 47–52.
Cassidy SB, McCandless SE : Prader-Willi syndrome; in Cassidy SB, Allanson JE (eds): Management of Genetic Syndromes. Hoboken, NJ: Wiley-Liss, 2005, pp 429–448.
Butler MG, Lee PDK, Whitman BY : Management of Prader-Willi Syndrome. Springer: New York, 2006.
Holm VA : Pipes: food and children with Prader-Willi syndrome. Am J Dis Child 1976; 130: 1063–1067.
Carrel AL, Lee PDK, Mogul HR : Growth hormone and Prader-Willi syndrome; in Butler MG Lee PDK, Whitman BY (eds): Management of Prader-Willi Syndrome, Springer: New York, 2006, pp 201–244.
Lindgren AC : Somatropin therapy for children with Prader-Willi syndrome: guidelines for use. Treat Endocrinol 2006; 5: 223–228.
Angulo MA, Castro-Magana M, Lamerson M, Arguello R, Accacha S, Khan A : Final adult height in children with Prader-Willi syndrome with and without human growth hormone treatment. Am J Med Genet A 2007; 143: 1456–1461.
Höybye C : Five-years growth hormone (GH) treatment in adults with Prader-Willi syndrome. Acta Paediatr 2007; 96: 410–413.
Mogul HR, Lee PDK, Whitman BY et al: Growth hormone treatment of adults with Prader-Willi syndrome and growth hormone deficiency improves lean body mass, fractional body fat, and serum triiodothyronine without glucose impairment: results from the US multi-center trial. J Clin Endo Metab 2008; 93: 1238–1245.
Miller J, Silverstein J, Shuster J, Driscoll DJ, Wagner M : Short-term effects of growth hormone on sleep abnormalities in Prader-Willi syndrome. J Clin Endocrinol Metab 2006; 91: 413–417.
Brice JA : Behavorial and psychotropic interventions in persons with Prader-Willi syndrome. Endocrinologist 2000; 10: S27–S30.
Dykens E, Shah B : Psychiatric disorders in Prader-Willi syndrome: epidemiology and management. CNS Drugs 2003; 17: 167–178.
Soni S, Whittington J, Holland AJ . et al: The course and outcome of psychiatric illness in people with Prader-Willi syndrome: implications for management and treatment. J Intellect Disabil Res 2007; 51: 32–42.
Kubota T, Aradhya S, Macha M et al: Analysis of parent of origin specific DNA methylation at SNRPN and PW71 in tissues: implication for prenatal diagnosis. J Med Genet 1996; 33: 1011–1014.
Ding F, Li HH, Zhang S et al: SnoRNA Snord116 (Pwcr1/MBII-85) deletion causes growth deficiency and hyperphagia in mice. PLoS ONE 2008; 3: e1709.
Further Reading
Cassidy SB, Schwartz S : Prader-Willi Syndrome; in: GeneReviews. Copyright, Seattle: University of Washington. updated March 2008. http://www.genetests.org.
Goldstone AP : Prader-Willi syndrome: advances in genetics, pathophysiology and treatment. Trends Endocrinol Metab 2004; 15: 12–20.
McCandless SE, Cassidy SB : 15q11-13 and the Prader-Willi syndrome; in: Epstein CJ, Erickson RP, Wynshaw-Boris A (eds): Molecular Basis of Inborn Errors of Development. Oxford University Press, 2008, 3rd edn, ch. 105.
Prader-Willi Syndrome Association (USA). website: www.pwsausa.org.
Acknowledgements
We thank Dr Charles Williams for his assistance with the figures, Dr Robert Nicholls for his helpful comments and the Hayward Foundation and NIH Grant U54RR019478 for funding support. We regret the omission of many deserving reference citations because of the limitation on the number of references.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cassidy, S., Driscoll, D. Prader–Willi syndrome. Eur J Hum Genet 17, 3–13 (2009). https://doi.org/10.1038/ejhg.2008.165
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejhg.2008.165
Keywords
This article is cited by
-
Scoliosis and rare diseases: our experience with the Prader–Willi syndrome
European Spine Journal (2024)
-
Autonomic nervous system dysfunction in Prader–Willi syndrome
Clinical Autonomic Research (2023)
-
Lebensqualität von kleinwüchsigen Kindern und Jugendlichen und Wachstumshormontherapie
Monatsschrift Kinderheilkunde (2023)
-
Biological, Behavioral, and Ethical Considerations of Prader-Willi Syndrome: A Primer for Behavior Analysts
Behavior Analysis in Practice (2022)
-
Suicidality in individuals with Prader-Willi syndrome: a review of registry survey data
BMC Psychiatry (2021)