Abstract
OBJECTIVE:
One developing strategy for obesity treatment has been to use combinations of differently acting pharmacotherapies to improve weight loss with fewer adverse effects. The purpose of this study was to determine whether the combination of naltrexone (Nal), an opioid antagonist acting on the reward system, and exendin-4 (Ex-4), a glucagon-like peptide 1 agonist acting on satiety signaling, would produce larger reductions in food intake than either alone in rats. Because the anorectic potencies of both compounds have been associated with nausea and malaise, the influence of these drug combinations on the acquisition of a conditioned taste aversion (CTA) was also determined.
METHODS:
In Experiment 1, the acute anorectic effects of Nal (0.32–3.2 mg kg−1; intraperitoneally (i.p.)) and Ex-4 (1–10 μg kg−1; i.p.) were assessed alone or in combination. Combinational doses were further investigated by the repeated daily administration of 1 mg kg−1 Nal+3.2 μg kg−1 Ex-4 for 4 days. In Experiment 2, both compounds alone or in combination were used as unconditioned stimuli in a series of CTA tests.
RESULTS:
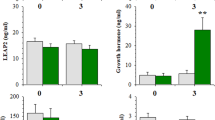
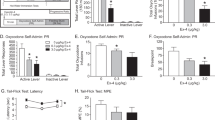
Nal and Ex-4, alone or in combination, suppressed food intake in a dose-dependent manner, and the interaction on food intake between Nal and Ex-4 was additive. In the CTA paradigm, Nal (1 mg kg−1) alone did not support acquisition, whereas a CTA was evident with doses of Ex-4 (1 or 3.2 μg kg−1). Combinations of Nal and Ex-4 also resulted in a more rapid and robust acquisition of a CTA.
CONCLUSION:
Given that the Nal and Ex-4 combination produces additive effects on not only food intake reduction but also food aversion learning, this specific drug combination does not have the benefit of minimizing the adverse effects associated with each individual drug. These data suggest that it is necessary to evaluate both the positive and adverse effects at early stages of combinational drug development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
WHO. Obesity and Overweight Factsheet. March, 2011, http://www.who.int/mediacentre/factsheets/fs311/en/index.html (accessed 26 September 2011).
Fontana MA, Wohlgemuth SD . The surgical treatment of metabolic disease and morbid obesity. Gastroenterol Clin North Am 2010; 39: 125–133.
Colquitt JL, Picot J, Loveman E, Clegg AJ . Surgery for obesity. Cochrane Database Syst Rev 2009; CD003641.
Picot J, Jones J, Colquitt JL, Gospodarevskaya E, Loveman E, Baxter L et al. The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation. Health Technol Assess 2009; 13: 1–190, 215–357, iii–iv.
Greenway FL, Bray GA . Combination drugs for treating obesity. Curr Diab Rep 2010; 10: 108–115.
Halford JC, Boyland EJ, Blundell JE, Kirkham TC, Harrold JA . Pharmacological management of appetite expression in obesity. Nat Rev Endocrinol 2010; 6: 255–269.
Greenway FL, Whitehouse MJ, Guttadauria M, Anderson JW, Atkinson RL, Fujioka K et al. Rational design of a combination medication for the treatment of obesity. Obesity (Silver Spring) 2009; 17: 30–39.
Vetter ML, Faulconbridge LF, Webb VL, Wadden TA . Behavioral and pharmacologic therapies for obesity. Nat Rev Endocrinol 2010; 6: 578–588.
Pohl M, Wank SA . Molecular cloning of the helodermin and exendin-4 cDNAs in the lizard. Relationship to vasoactive intestinal polypeptide/pituitary adenylate cyclase activating polypeptide and glucagon-like peptide 1 and evidence against the existence of mammalian homologues. J Biol Chem 1998; 273: 9778–9784.
Raufman JP, Jensen RT, Sutliff VE, Pisano JJ, Gardner JD . Actions of Gila monster venom on dispersed acini from guinea pig pancreas. Am J Physiol 1982; 242: G470–G474.
Blonde L, Klein EJ, Han J, Zhang B, Mac SM, Poon TH et al. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab 2006; 8: 436–447.
Klonoff DC, Buse JB, Nielsen LL, Guan X, Bowlus CL, Holcombe JH et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin 2008; 24: 275–286.
Fantino M, Hosotte J, Apfelbaum M . An opioid antagonist, naltrexone, reduces preference for sucrose in humans. Am J Physiol 1986; 251: R91–R96.
Taha SA, Norsted E, Lee LS, Lang PD, Lee BS, Woolley JD et al. Endogenous opioids encode relative taste preference. Eur J Neurosci 2006; 24: 1220–1226.
Yeomans MR, Gray RW . Selective effects of naltrexone on food pleasantness and intake. Physiol Behav 1996; 60: 439–446.
Parker LA, Rennie M . Naltrexone-induced aversions: assessment by place conditioning, taste reactivity, and taste avoidance paradigms. Pharmacol Biochem Behav 1992; 41: 559–565.
Srisurapanont M, Jarusuraisin N . Naltrexone for the treatment of alcoholism: a meta-analysis of randomized controlled trials. Int J Neuropsychopharmacol 2005; 8: 267–280.
Norgren R, Grigson P, Hajnal A, Lundy RFJ . Motivational modulation of taste. In: Ono T et al. (eds). Limbic and Association Cortical Systems: Basic, Clinical and Computational Aspects. Elsevier Science: Amsterdam, 2003, pp 319–334.
Mungarndee SS, Lundy Jr RF, Norgren R . Central gustatory lesions and learned taste aversions: unconditioned stimuli. Physiol Behav 2006; 87: 542–551.
Scalera G, Grigson PS, Norgren R . Gustatory functions, sodium appetite, and conditioned taste aversion survive excitotoxic lesions of the thalamic taste area. Behav Neurosci 1997; 111: 633–645.
Bello NT, Kemm MH, Ofeldt EM, Moran TH . Dose combinations of exendin-4 and salmon calcitonin produce additive and synergistic reductions in food intake in nonhuman primates. Am J Physiol Regul Integr Comp Physiol 2010; 299: R945–R952.
Gennings C, Carter Jr WH, Campbell ED, Staniswalis JG, Martin TJ, Martin BR et al. Isobolographic characterization of drug interactions incorporating biological variability. J Pharmacol Exp Ther 1990; 252: 208–217.
Roth JD, Trevaskis JL, Turek VF, Parkes DG . ‘Weighing in’ on synergy: preclinical research on neurohormonal anti-obesity combinations. Brain Res 2010; 1350: 86–94.
Trevaskis JL, Coffey T, Cole R, Lei C, Wittmer C, Walsh B et al. Amylin-mediated restoration of leptin responsiveness in diet-induced obesity: magnitude and mechanisms. Endocrinology 2008; 149: 5679–5687.
Roth JD, Coffey T, Jodka CM, Maier H, Athanacio JR, Mack CM et al. Combination therapy with amylin and peptide YY[3-36] in obese rodents: anorexigenic synergy and weight loss additivity. Endocrinology 2007; 148: 6054–6061.
Tallarida RJ, Raffa RB . The application of drug dose equivalence in the quantitative analysis of receptor occupation and drug combinations. Pharmacol Ther 2010; 127: 165–174.
Baldo BA, Pratt WE, Kelley AE . Control of Fat Intake by Striatal Opioids. In: Montmayeur JP, le Coutre J (eds). Fat Detection: Taste, Texture, and Post Ingestive Effects. Chapter 13. CRC Press: Boca Raton, FL, 2010.
Taha SA . Preference or fat? Revisiting opioid effects on food intake. Physiol Behav 2010; 100: 429–437.
Glass MJ, Billington CJ, Levine AS . Naltrexone administered to central nucleus of amygdala or PVN: neural dissociation of diet and energy. Am J Physiol Regul Integr Comp Physiol 2000; 279: R86–R92.
Atkinson RL, Berke LK, Drake CR, Bibbs ML, Williams FL, Kaiser DL . Effects of long-term therapy with naltrexone on body weight in obesity. Clin Pharmacol Ther 1985; 38: 419–422.
Malcolm R, O’Neil PM, Sexauer JD, Riddle FE, Currey HS, Counts C . A controlled trial of naltrexone in obese humans. Int J Obes 1985; 9: 347–353.
Alhadeff AL, Rupprecht LE, Hayes MR . GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology 2012; 153: 647–658.
Dossat AM, Lilly N, Kay K, Williams DL . Glucagon-like peptide 1 receptors in nucleus accumbens affect food intake. J Neurosci 2011; 31: 14453–14457.
Freeman JS . Optimizing outcomes for GLP-1 agonists. J Am Osteopath Assoc 2011; 111: eS15–eS20.
Han VK, Hynes MA, Jin C, Towle AC, Lauder JM, Lund PK . Cellular localization of proglucagon/glucagon-like peptide I messenger RNAs in rat brain. J Neurosci Res 1986; 16: 97–107.
Shimizu I, Hirota M, Ohboshi C, Shima K . Identification and localization of glucagon-like peptide-1 and its receptor in rat brain. Endocrinology 1987; 121: 1076–1082.
Uttenthal LO, Toledano A, Blazquez E . Autoradiographic localization of receptors for glucagon-like peptide-1 (7-36) amide in rat brain. Neuropeptides 1992; 21: 143–146.
Sarkar S, Fekete C, Legradi G, Lechan RM . Glucagon like peptide-1 (7-36) amide (GLP-1) nerve terminals densely innervate corticotropin-releasing hormone neurons in the hypothalamic paraventricular nucleus. Brain Res 2003; 985: 163–168.
Kinzig KP, D’Alessio DA, Seeley RJ . The diverse roles of specific GLP-1 receptors in the control of food intake and the response to visceral illness. J Neurosci 2002; 22: 10470–10476.
Thiele TE, Van Dijk G, Campfield LA, Smith FJ, Burn P, Woods SC et al. Central infusion of GLP-1, but not leptin, produces conditioned taste aversions in rats. Am J Physiol 1997; 272: R726–R730.
Seeley RJ, Blake K, Rushing PA, Benoit S, Eng J, Woods SC et al. The role of CNS glucagon-like peptide-1 (7-36) amide receptors in mediating the visceral illness effects of lithium chloride. J Neurosci 2000; 20: 1616–1621.
Rinaman L . Interoceptive stress activates glucagon-like peptide-1 neurons that project to the hypothalamus. Am J Physiol 1999; 277: R582–R590.
Kanoski SE, Fortin SM, Arnold M, Grill HJ, Hayes MR . Peripheral and central GLP-1 receptor populations mediate the anorectic effects of Peripherally administered GLP-1 receptor Agonists, liraglutide and exendin-4. Endocrinology 2011; 152: 3103–3112.
Williams DL, Baskin DG, Schwartz MW . Evidence that intestinal glucagon-like peptide-1 plays a physiological role in satiety. Endocrinology 2009; 150: 1680–1687.
Goke R, Larsen PJ, Mikkelsen JD, Sheikh SP . Distribution of GLP-1 binding sites in the rat brain: evidence that exendin-4 is a ligand of brain GLP-1 binding sites. Eur J Neurosci 1995; 7: 2294–2300.
Kastin AJ, Akerstrom V . Entry of exendin-4 into brain is rapid but may be limited at high doses. Int J Obes Relat Metab Disord 2003; 27: 313–318.
Hayes MR, Bradley L, Grill HJ . Endogenous hindbrain glucagon-like peptide-1 receptor activation contributes to the control of food intake by mediating gastric satiation signaling. Endocrinology 2009; 150: 2654–2659.
De Jonghe BC, Lawler MP, Horn CC, Tordoff MG . Pica as an adaptive response: Kaolin consumption helps rats recover from chemotherapy-induced illness. Physiol Behav 2009; 97: 87–90.
Horn CC, De Jonghe BC, Matyas K, Norgren R . Chemotherapy-induced kaolin intake is increased by lesion of the lateral parabrachial nucleus of the rat. Am J Physiol Regul Integr Comp Physiol 2009; 297: R1375–R1382.
Mack CM, Moore CX, Jodka CM, Bhavsar S, Wilson JK, Hoyt JA et al. Antiobesity action of peripheral exenatide (exendin-4) in rodents: effects on food intake, body weight, metabolic status and side-effect measures. Int J Obes (Lond) 2006; 30: 1332–1340.
Baraboi ED, St-Pierre DH, Shooner J, Timofeeva E, Richard D . Brain activation following peripheral administration of the Glp-1 receptor agonist exendin-4. Am J Physiol Regul Integr Comp Physiol 2011; 301: R1011–R1024.
Kanoski SE, Rupprecht LE, Fortin SM, De Jonghe BC, Hayes MR . The role of nausea in food intake and body weight suppression by peripheral GLP-1 receptor agonists, exendin-4 and liraglutide. Neuropharmacology 2011; e-pub ahead of print 28 December 2011.
Talsania T, Anini Y, Siu S, Drucker DJ, Brubaker PL . Peripheral exendin-4 and peptide YY(3-36) synergistically reduce food intake through different mechanisms in mice. Endocrinology 2005; 146: 3748–3756.
Scott KA, Moran TH . The GLP-1 agonist exendin-4 reduces food intake in nonhuman primates through changes in meal size. Am J Physiol Regul Integr Comp Physiol 2007; 293: R983–R987.
Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD . Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care 2004; 27: 2628–2635.
Acknowledgements
We thank Ryan Purcell, Drs Gretha Boersma and Yada Tresssukosol for assistance with animal care and CTA experiments. This research was supported by the National Institutes of Health grant DK19302.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Liang, NC., Bello, N. & Moran, T. Additive feeding inhibitory and aversive effects of naltrexone and exendin-4 combinations. Int J Obes 37, 272–278 (2013). https://doi.org/10.1038/ijo.2012.16
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2012.16
Keywords
This article is cited by
-
Exendin-4 reduces food intake via the PI3K/AKT signaling pathway in the hypothalamus
Scientific Reports (2017)
-
Effects of peripheral administration of a Neuromedin U receptor 2-selective agonist on food intake and body weight in obese mice
International Journal of Obesity (2017)
-
Behavioural profile of exendin-4/naltrexone dose combinations in male rats during tests of palatable food consumption
Psychopharmacology (2014)
-
Opposing neural effects of naltrexone on food reward and aversion: implications for the treatment of obesity
Psychopharmacology (2014)