Abstract
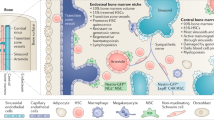
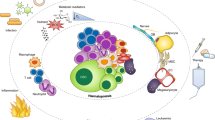
In post-fetal life, hematopoiesis occurs in unique microenvironments or ‘niches’ in the marrow. Niches facilitate the maintenance of hematopoietic stem cells (HSCs) as unipotent, while supporting lineage commitment of the expanding blood populations. As the physical locale that regulates HSC function, the niche function is vitally important to the survival of the organism. This places considerable selective pressure on HSCs, as only those that are able to engage the niche in the appropriate context are likely to be maintained as stem cells. Since niches are central regulators of stem cell function, it is not surprising that molecular parasites like neoplasms are likely to seek out opportunities to harvest resources from the niche environment. As such, the niche may unwittingly participate in tumorigenesis as a leukemic or neoplastic niche. The niche may also promote metastasis or chemo-resistance of hematogenous neoplasms or solid tumors. This review focuses on what is known about the physical structures of the niche, how the niche participates in hematopoiesis and neoplastic growth and what molecules are involved. Further understanding of the interactions between stem cells and the niche may be useful for developing therapeutic strategies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Grinnell J . The niche relationships of the California thrasher. Auk 1917; 34: 427–433.
Hutchinson GE . Concluding remarks. Cold Spring Harb Symp Quant Biol 1957; 22: 415–427.
Schofield R . The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells 1978; 4: 7–25.
Miura Y, Gao Z, Miura M, Seo BM, Sonoyama W, Chen W et al. Mesenchymal stem cell-organized bone marrow elements: an alternative hematopoietic progenitor resource. Stem Cells 2006; 24: 2428–2436.
Taichman RS . Blood and bone: two tissues whose fates are intertwined to create the hematopoietic stem-cell niche. Blood 2005; 105: 2631–2639.
Patt HM, Maloney MA . Bone formation and resorption as a requirement for marrow development. Proc Soc Exp Biol Med 1972; 140: 205–207.
Lord BI, Hendry JH . The distribution of haemopoietic colony-forming units in the mouse femur, and its modification by X-rays. Br J Radiol 1972; 45: 110–115.
Taichman RS, Emerson SG . Human osteoblasts support hematopoiesis through the production of granulocyte colony-stimulating factor. J Exp Med 1994; 179: 1677–1682.
Zhang J, Niu C, Ye L, Huang H, He X, Tong WG et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature 2003; 425: 836–841.
Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 2003; 425: 841–846.
Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell 2004; 118: 149–161.
Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C, Morrison SJ . SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 2005; 121: 1109–1121.
Dar A, Goichberg P, Shinder V, Kalinkovich A, Kollet O, Netzer N et al. Chemokine receptor CXCR4-dependent internalization and resecretion of functional chemokine SDF-1 by bone marrow endothelial and stromal cells. Nat Immunol 2005; 6: 1038–1046.
Chute JP, Muramoto GG, Dressman HK, Wolfe G, Chao NJ, Lin S . Molecular profile and partial functional analysis of novel endothelial cell-derived growth factors that regulate hematopoiesis. Stem Cells 2006; 24: 1315–1327.
Petit I, Jin D, Rafii S . The SDF-1-CXCR4 signaling pathway: a molecular hub modulating neo-angiogenesis. Trends Immunol 2007; 28: 299–307.
Balduino A, Hurtado SP, Frazao P, Takiya CM, Alves LM, Nasciutti LE et al. Bone marrow subendosteal microenvironment harbours functionally distinct haemosupportive stromal cell populations. Cell Tissue Res 2005; 319: 255–266.
Neiva K, Sun YX, Taichman RS . The role of osteoblasts in regulating hematopoietic stem cell activity and tumor metastasis. Braz J Med Biol Res 2005; 38: 1449–1454.
Cooper CR, Chay CH, Gendernalik JD, Lee HL, Bhatia J, Taichman RS et al. Stromal factors involved in prostate carcinoma metastasis to bone. Cancer 2003; 97 (3 Suppl): 739–747.
Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman NS, McCauley LK . Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res 2002; 62: 1832–1837.
Sun YX, Wang J, Shelburne CE, Lopatin DE, Chinnaiyan AM, Rubin MA et al. Expression of CXCR4 and CXCL12 (SDF-1) in human prostate cancers (PCa) in vivo. J Cell Biochem 2003; 89: 462–473.
Sun YX, Schneider A, Jung Y, Wang J, Dai J, Cook K et al. Skeletal localization and neutralization of the SDF-1(CXCL12)/CXCR4 axis blocks prostate cancer metastasis and growth in osseous sites in vivo. J Bone Miner Res 2005; 20: 318–329.
Havens AM, Jung Y, Sun YX, Wang J, Shah RB, Buhring HJ et al. The role of sialomucin CD164 (MGC-24v or endolyn) in prostate cancer metastasis. BMC Cancer 2006; 6: 195.
Sun YX, Fang M, Wang J, Cooper CR, Pienta KJ, Taichman RS . Expression and activation of alpha(v)beta(3) integrins by SDF-1/CXC12 increases the aggressiveness of prostate cancer cells. Prostate 2007; 67: 61–73.
Bewick MA, Lafrenie RM . Adhesion dependent signalling in the tumour microenvironment: the future of drug targetting. Curr Pharm Des 2006; 12: 2833–2848.
Li ZW, Dalton WS . Tumor microenvironment and drug resistance in hematologic malignancies. Blood Rev 2006; 20: 333–342.
Ramasamy R, Lam EW, Soeiro I, Tisato V, Bonnet D, Dazzi F . Mesenchymal stem cells inhibit proliferation and apoptosis of tumor cells: impact on in vivo tumor growth. Leukemia 2007; 21: 304–310.
Ninomiya M, Abe A, Katsumi A, Xu J, Ito M, Arai F et al. Homing, proliferation and survival sites of human leukemia cells in vivo in immunodeficient mice. Leukemia 2007; 21: 136–142.
Kollet O, Dar A, Shivtiel S, Kalinkovich A, Lapid K, Sztainberg Y et al. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med 2006; 12: 657–664.
Taichman RS, Emerson SG . The role of osteoblasts in the hematopoietic microenvironment. Stem Cells 1998; 16: 7–15.
Taichman RS, Reilly MJ, Emerson SG . Human osteoblasts support human hematopoietic progenitor cells in vitro bone marrow cultures. Blood 1996; 87: 518–524.
Taichman RS, Reilly MJ, Emerson SG . Human osteosarcomas inhibit hematopoietic colony formation: partial reversal by antibody to transforming growth factor-beta 1. Bone 1997; 21: 353–361.
Taichman R, Reilly M, Verma R, Ehrenman K, Emerson S . Hepatocyte growth factor is secreted by osteoblasts and cooperatively permits the survival of haematopoietic progenitors. Br J Haematol 2001; 112: 438–448.
Taichman RS, Reilly MJ, Verma RS, Emerson SG . Augmented production of interleukin-6 by normal human osteoblasts in response to CD34+ hematopoietic bone marrow cells in vitro. Blood 1997; 89: 1165–1172.
Taichman RS, Reilly MJ, Matthews LS . Human osteoblast-like cells and osteosarcoma cell lines synthesize macrophage inhibitory protein 1alpha in response to interleukin 1beta and tumour necrosis factor alpha stimulation in vitro. Br J Haematol 2000; 108: 275–283.
Zhu J, Garrett R, Jung Y, Zhang Y, Kim N, Wang J et al. Osteoblasts support B-lymphocyte commitment and differentiation from hematopoietic stem cells. Blood 2007; 109: 3706–3712.
Crean SM, Meneski JP, Hullinger TG, Reilly MJ, DeBoever EH, Taichman RS . N-linked sialyated sugar receptors support haematopoietic cell-osteoblast adhesions. Br J Haematol 2004; 124: 534–546.
Ponomaryov T, Peled A, Petit I, Taichman RS, Habler L, Sandbank J et al. Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. J Clin Invest 2000; 106: 1331–1339.
Jung Y, Wang J, Schneider A, Sun YX, Koh-Paige AJ, Osman NI et al. Regulation of SDF-1 (CXCL12) production by osteoblasts; a possible mechanism for stem cell homing. Bone 2006; 38: 497–508.
Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol 2002; 3: 687–694.
Burger JA, Kipps TJ . CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood 2006; 107: 1761–1767.
Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature 1996; 382: 635–638.
Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T et al. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci USA 1998; 95: 9448–9453.
Tachibana K, Hirota S, Iizasa H, Yoshida H, Kawabata K, Kataoka Y et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature 1998; 393: 591–594.
Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR . Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature 1998; 393: 595–599.
Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science 1999; 283: 845–848.
Sugiyama T, Kohara H, Noda M, Nagasawa T . Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 2006; 25: 977–988.
Jung Y, Wang J, Song J, Shiozawa Y, Havens A, Wang Z et al. Annexin II expressed by osteoblasts and endothelial cells regulates stem cell adhesion, homing, and engraftment following transplantation. Blood 2007; 110: 82–90.
Nilsson SK, Johnston HM, Whitty GA, Williams B, Webb RJ, Denhardt DT et al. Osteopontin, a key component of the hematopoietic stem cell niche and regulator of primitive hematopoietic progenitor cells. Blood 2005; 106: 1232–1239.
Stier S, Ko Y, Forkert R, Lutz C, Neuhaus T, Grunewald E et al. Osteopontin is a hematopoietic stem cell niche component that negatively regulates stem cell pool size. J Exp Med 2005; 201: 1781–1791.
Jung Y, Wang J, Havens A, Sun Y, Jin T, Taichman RS . Cell-to-cell contact is critical for the survival of hematopoietic progenitor cells on osteoblasts. Cytokine 2005; 32: 155–162.
Kiel MJ, Radice GL, Morrison SJ . Lack of evidence that hematopoietic stem cells depend on N-cadherin-mediated adhesion to osteoblasts for their maintenance. Cell Stem Cell 2007; 1: 204–217.
Driessen RL, Johnston HM, Nilsson SK . Membrane-bound stem cell factor is a key regulator in the initial lodgment of stem cells within the endosteal marrow region. Exp Hematol 2003; 31: 1284–1291.
Adams GB, Chabner KT, Alley IR, Olson DP, Szczepiorkowski ZM, Poznansky MC et al. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature 2006; 439: 599–603.
Wilson A, Murphy MJ, Oskarsson T, Kaloulis K, Bettess MD, Oser GM et al. c-Myc controls the balance between hematopoietic stem cell self-renewal and differentiation. Genes Dev 2004; 18: 2747–2763.
Avecilla ST, Hattori K, Heissig B, Tejada R, Liao F, Shido K et al. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat Med 2004; 10: 64–71.
Yin T, Li L . The stem cell niches in bone. J Clin Invest 2006; 116: 1195–1201.
Castro-Malaspina H, Gay RE, Resnick G, Kapoor N, Meyers P, Chiarieri D et al. Characterization of human bone marrow fibroblast colony-forming cells (CFU-F) and their progeny. Blood 1980; 56: 289–301.
Beresford JN . Osteogenic stem cells and the stromal system of bone and marrow. Clin Orthop 1989; 240: 270–280.
Cassiede P, Dennis JE, Ma F, Caplan AI . Osteochondrogenic potential of marrow mesenchymal progenitor cells exposed to TGF-beta 1 or PDGF-BB as assayed in vivo and in vitro. J Bone Miner Res 1996; 11: 1264–1273.
Chen JL, Hunt P, McElvain M, Black T, Kaufman S, Choi ES . Osteoblast precursor cells are found in CD34+ cells from human bone marrow. Stem Cells 1997; 15: 368–377.
Majumdar MK, Thiede MA, Mosca JD, Moorman M, Gerson SL . Phenotypic and functional comparison of cultures of marrow-derived mesenchymal stem cells (MSCs) and stromal cells. J Cell Physiol 1998; 176: 57–66.
Eipers PG, Kale S, Taichman RS, Pipia GG, Swords NA, Mann KG et al. Bone marrow accessory cells regulate human bone precursor cell development. Exp Hematol 2000; 28: 815–825.
Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 2002; 418: 41–49.
Kuznetsov SA, Mankani MH, Gronthos S, Satomura K, Bianco P, Robey PG . Circulating skeletal stem cells. J Cell Biol 2001; 153: 1133–1140.
Prockop DJ . Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 1997; 276: 71–74.
Bianco P, Robey PG . Stem cells in tissue engineering. Nature 2001; 414: 118–121.
Gronthos S, Zannettino AC, Hay SJ, Shi S, Graves SE, Kortesidis A et al. Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J Cell Sci 2003; 116 (Part 9): 1827–1835.
Smith JR, Pochampally R, Perry A, Hsu SC, Prockop DJ . Isolation of a highly clonogenic and multipotential subfraction of adult stem cells from bone marrow stroma. Stem Cells 2004; 22: 823–831.
Falla N, Van V, Bierkens J, Borremans B, Schoeters G, Van Gorp U . Characterization of a 5-fluorouracil-enriched osteoprogenitor population of the murine bone marrow. Blood 1993; 82: 3580–3591.
Baksh D, Yao R, Tuan RS . Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells 2007; 25: 1384–1392.
Wang Z, Song J, Taichman RS, Krebsbach PH . Ablation of proliferating marrow with 5-fluorouracil allows partial purification of mesenchymal stem cells. Stem Cells 2006; 24: 1573–1582.
Kucia M, Reca R, Campbell FR, Zuba-Surma E, Majka M, Ratajczak J et al. A population of very small embryonic-like (VSEL) CXCR4(+)SSEA-1(+)Oct-4+ stem cells identified in adult bone marrow. Leukemia 2006; 20: 857–869.
Kucia M, Zuba-Surma EK, Wysoczynski M, Wu W, Ratajczak J, Machalinski B et al. Adult marrow-derived very small embryonic-like stem cells and tissue engineering. Expert Opin Biol Ther 2007; 7: 1499–1514.
Kucia M, Ratajczak J, Reca R, Janowska-Wieczorek A, Ratajczak MZ . Tissue-specific muscle, neural and liver stem/progenitor cells reside in the bone marrow, respond to an SDF-1 gradient and are mobilized into peripheral blood during stress and tissue injury. Blood Cells Mol Dis 2004; 32: 52–57.
Ratajczak MZ, Kucia M, Reca R, Majka M, Janowska-Wieczorek A, Ratajczak J . Stem cell plasticity revisited: CXCR4-positive cells expressing mRNA for early muscle, liver and neural cells ‘hide out’ in the bone marrow. Leukemia 2004; 18: 29–40.
Kucia M, Wu W, Ratajczak MZ . Bone marrow-derived very small embryonic-like stem cells: their developmental origin and biological significance. Dev Dyn 2007; 236: 3309–3320.
Ratajczak MZ, Machalinski B, Wojakowski W, Ratajczak J, Kucia M . A hypothesis for an embryonic origin of pluripotent Oct-4(+) stem cells in adult bone marrow and other tissues. Leukemia 2007; 21: 860–867.
Ratajczak J, Kucia M, Zuba-Surma E, Reca R, Ratajczak MZ . The CD45(−)lin(−) adult marrow-derived CXCR4(+) SSEA-1(+) Oct-4(+) very small embryonic-like (VSEL) stem cells form in vitro spheres which may differentiate into CD45(+) hematopoietic cells. Blood 2006; 108: 86A.
D’Ippolito G, Diabira S, Howard GA, Menei P, Roos BA, Schiller PC . Marrow-isolated adult multilineage inducible (MIAMI) cells, a unique population of postnatal young and old human cells with extensive expansion and differentiation potential. J Cell Sci 2004; 117 (Part 14): 2971–2981.
Devine SM, Hoffman R . Role of mesenchymal stem cells in hematopoietic stem cell transplantation. Curr Opin Hematol 2000; 7: 358–363.
Lazarus HM, Koc ON, Devine SM, Curtin P, Maziarz RT, Holland HK et al. Cotransplantation of HLA-identical sibling culture-expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients. Biol Blood Marrow Transplant 2005; 11: 389–398.
Ball LM, Bernardo ME, Roelofs H, Lankester A, Cometa A, Egeler RM et al. Cotransplantation of ex vivo expanded mesenchymal stem cells accelerates lymphocyte recovery and may reduce the risk of graft failure in haploidentical hematopoietic stem-cell transplantation. Blood 2007; 110: 2764–2767.
Kadereit S, Deeds LS, Haynesworth SE, Koc ON, Kozik MM, Szekely E et al. Expansion of LTC-ICs and maintenance of p21 and BCL-2 expression in cord blood CD34(+)/CD38(−) early progenitors cultured over human MSCs as a feeder layer. Stem Cells 2002; 20: 573–582.
Koh SH, Choi HS, Park ES, Kang HJ, Ahn HS, Shin HY . Co-culture of human CD34+ cells with mesenchymal stem cells increases the survival of CD34+ cells against the 5-aza-deoxycytidine- or trichostatin A-induced cell death. Biochem Biophys Res Commun 2005; 329: 1039–1045.
Li N, Feugier P, Serrurrier B, Latger-Cannard V, Lesesve JF, Stoltz JF et al. Human mesenchymal stem cells improve ex vivo expansion of adult human CD34+ peripheral blood progenitor cells and decrease their allostimulatory capacity. Exp Hematol 2007; 35: 507–515.
Pedemonte E, Benvenuto F, Casazza S, Mancardi G, Oksenberg JR, Uccelli A et al. The molecular signature of therapeutic mesenchymal stem cells exposes the architecture of the hematopoietic stem cell niche synapse. BMC Genomics 2007; 8: 65.
Deans RJ, Moseley AB . Mesenchymal stem cells: biology and potential clinical uses. Exp Hematol 2000; 28: 875–884.
Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F . Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood 2005; 105: 2821–2827.
Benvenuto F, Ferrari S, Gerdoni E, Gualandi F, Frassoni F, Pistoia V et al. Human mesenchymal stem cells promote survival of T cells in a quiescent state. Stem Cells 2007; 25: 1753–1760.
Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F et al. Human mesenchymal stem cells modulate B-cell functions. Blood 2006; 107: 367–372.
Maccario R, Podesta M, Moretta A, Cometa A, Comoli P, Montagna D et al. Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4+ T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica 2005; 90: 516–525.
Ramasamy R, Fazekasova H, Lam EW, Soeiro I, Lombardi G, Dazzi F . Mesenchymal stem cells inhibit dendritic cell differentiation and function by preventing entry into the cell cycle. Transplantation 2007; 83: 71–76.
Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 2007; 449: 557–563.
Bonnet D, Dick JE . Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med 1997; 3: 730–737.
Shipitsin M, Campbell LL, Argani P, Weremowicz S, Bloushtain-Qimron N, Yao J et al. Molecular definition of breast tumor heterogeneity. Cancer Cell 2007; 11: 259–273.
Keller ET, Zhang J, Cooper CR, Smith PC, McCauley LK, Pienta KJ et al. Prostate carcinoma skeletal metastases: cross-talk between tumor and bone. Cancer Metastasis Rev 2001; 20: 333–349.
Wang J, Sun Y, Song W, Nor JE, Wang CY, Taichman RS . Diverse signaling pathways through the SDF-1/CXCR4 chemokine axis in prostate cancer cell lines leads to altered patterns of cytokine secretion and angiogenesis. Cell Signal 2005; 17: 1578–1592.
Dai J, Kitagawa Y, Zhang J, Yao Z, Mizokami A, Cheng S et al. Vascular endothelial growth factor contributes to the prostate cancer-induced osteoblast differentiation mediated by bone morphogenetic protein. Cancer Res 2004; 64: 994–999.
Hullinger TG, Taichman RS, Linseman DA, Somerman MJ . Secretory products from PC-3 and MCF-7 tumor cell lines upregulate osteopontin in MC3T3-E1 cells. J Cell Biochem 2000; 78: 607–616.
Ravandi F, Estrov Z . Eradication of leukemia stem cells as a new goal of therapy in leukemia. Clin Cancer Res 2006; 12: 340–344.
Mudry RE, Fortney JE, York T, Hall BM, Gibson LF . Stromal cells regulate survival of B-lineage leukemic cells during chemotherapy. Blood 2000; 96: 1926–1932.
Iwamoto S, Mihara K, Downing JR, Pui CH, Campana D . Mesenchymal cells regulate the response of acute lymphoblastic leukemia cells to asparaginase. J Clin Invest 2007; 117: 1049–1057.
Nefedova Y, Landowski TH, Dalton WS . Bone marrow stromal-derived soluble factors and direct cell contact contribute to de novo drug resistance of myeloma cells by distinct mechanisms. Leukemia 2003; 17: 1175–1182.
Gazitt Y . Homing and mobilization of hematopoietic stem cells and hematopoietic cancer cells are mirror image processes, utilizing similar signaling pathways and occurring concurrently: circulating cancer cells constitute an ideal target for concurrent treatment with chemotherapy and antilineage-specific antibodies. Leukemia 2004; 18: 1–10.
Vincent T, Mechti N . Extracellular matrix in bone marrow can mediate drug resistance in myeloma. Leuk Lymphoma 2005; 46: 803–811.
Wang L, O’Leary H, Fortney J, Gibson LF . Ph+/VE-cadherin+ identifies a stem cell like population of acute lymphoblastic leukemia sustained by bone marrow niche cells. Blood 2007; 110: 3334–3344.
Matsunaga T, Takemoto N, Sato T, Takimoto R, Tanaka I, Fujimi A et al. Interaction between leukemic-cell VLA-4 and stromal fibronectin is a decisive factor for minimal residual disease of acute myelogenous leukemia. Nat Med 2003; 9: 1158–1165.
Jin L, Hope KJ, Zhai Q, Smadja-Joffe F, Dick JE . Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med 2006; 12: 1167–1174.
Sipkins DA, Wei X, Wu JW, Runnels JM, Cote D, Means TK et al. In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature 2005; 435: 969–973.
Veiga JP, Costa LF, Sallan SE, Nadler LM, Cardoso AA . Leukemia-stimulated bone marrow endothelium promotes leukemia cell survival. Exp Hematol 2006; 34: 610–621.
Rizo A, Vellenga E, de Haan G, Schuringa JJ . Signaling pathways in self-renewing hematopoietic and leukemic stem cells: do all stem cells need a niche? Hum Mol Genet 2006; 15: R210–R219.
Buzzeo MP, Scott EW, Cogle CR . The hunt for cancer-initiating cells: a history stemming from leukemia. Leukemia 2007; 21: 1619–1627.
Oakley EJ, Van Zant G . Unraveling the complex regulation of stem cells: implications for aging and cancer. Leukemia 2007; 21: 612–621.
Acknowledgements
We are grateful to Chris Jung for his artistic renditions and to Dr Yuri Shiozawa for editorial assistance. We also gratefully acknowledge Professor Joerg Huelsken (Swiss Institute for Experimental Cancer Research, Epalinges, Switzerland) for inspiration for Figure 3. This work was directly supported by a Pediatric Oncology Research Fellowship (Y Shiozawa), CA93900 (RS Taichman and KJ Pienta), Department of Defense PC060857 (RS Taichman), P50 CA69568 (KJ Pienta), U19 CA113317 (KJ Pienta) and 2006 and 2007 awards from the Prostate Cancer Foundation (RS Taichman and KJ Pienta).
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclosure/Conflict of interest
KJ Pienta receives support as an American Cancer Society Clinical Research professor.
Rights and permissions
About this article
Cite this article
Shiozawa, Y., Havens, A., Pienta, K. et al. The bone marrow niche: habitat to hematopoietic and mesenchymal stem cells, and unwitting host to molecular parasites. Leukemia 22, 941–950 (2008). https://doi.org/10.1038/leu.2008.48
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2008.48
Keywords
This article is cited by
-
Extracellular vesicle-mediated remodeling of the bone marrow microenvironment in myeloid malignancies
International Journal of Hematology (2023)
-
Direct bone marrow injection of human bone marrow-derived stromal cells into mouse femurs results in greater prostate cancer PC-3 cell proliferation, but not specifically proliferation within the injected femurs
BMC Cancer (2022)
-
Human bone marrow-derived stromal cell behavior when injected directly into the bone marrow of NOD-scid-gamma mice pre-conditioned with sub-lethal irradiation
Stem Cell Research & Therapy (2021)
-
Bone marrow niches in the regulation of bone metastasis
British Journal of Cancer (2021)
-
Various pathways of zoledronic acid against osteoclasts and bone cancer metastasis: a brief review
BMC Cancer (2020)