Abstract
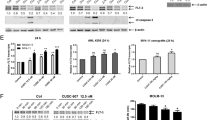
We report the results of a phase I dose escalation trial of the multikinase inhibitor sorafenib in relapsed and refractory acute leukemia patients using an intermittent dosing regimen. Fifteen patients with advanced leukemia (12 with acute myeloid leukemia, 2 with acute lymphoblastic leukemia, 1 with biphenotypic) and a median age of 63 (range 37–85) years were enrolled and treated on a dose escalation trial. Toxicities ⩾grade 3 were present in 55% of cycles and the maximum tolerated dose (MTD) was determined to be 400 mg b.i.d. × 21days in a 28-day cycle. Plasma inhibitory assays of kinase targets extracellular signal-regulated kinase (ERK) and FLT3-internal tandem duplication (ITD) showed excellent target inhibition, with FLT3-ITD silencing occurring below the MTD. The N-oxide metabolite of sorafenib seemed to be a more potent inhibitor of FLT3-ITD than the parent compound. Despite marked ex vivo FLT-3 ITD inhibition, no patients met the criteria for complete or partial response in this monotherapy study. Out of 15 patients, 11 experienced stable disease as best response. Although sorafenib showed only modest clinical activity as a single agent in this heavily treated population, robust inhibition of FLT3 and ERK suggests that there may be a potential important role in combination therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Larson S, Stock W . Progress in the treatment of adults with acute lymphoblastic leukemia. Curr Opin Hematol 2008; 15: 400–407.
Tallman MS, Gilliland DG, Rowe JM . Drug therapy for acute myeloid leukemia. Blood 2005; 106: 1154–1163.
Ricciardi MR, McQueen T, Chism D, Milella M, Estey E, Kaldjian E et al. Quantitative single cell determination of ERK phosphorylation and regulation in relapsed and refractory primary acute myeloid leukemia. Leukemia 2005; 19: 1543–1549.
Kornblau SM, Womble M, Qiu YH, Jackson CE, Chen W, Konopleva M et al. Simultaneous activation of multiple signal transduction pathways confers poor prognosis in acute myelogenous leukemia. Blood 2006; 108: 2358–2365.
Hilger RA, Scheulen ME, Strumberg D . The Ras-Raf-MEK-ERK pathway in the treatment of cancer. Onkologie 2002; 25: 511–518.
Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the raf/mek/erk pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 2004; 64: 7099–7109.
Kane RC, Farrell AT, Saber H, Tang S, Williams G, Jee JM et al. Sorafenib for the treatment of advanced renal cell carcinoma. Clin Cancer Res 2006; 12: 7271–7278.
Abou-Alfa GK, Schwartz L, Ricci S, Amadori D, Santoro A, Figer A et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol 2006; 24: 4293–4300.
Yu C, Bruzek LM, Meng XW, Gores GJ, Carter CA, Kaufmann SH et al. The role of Mcl-1 downregulation in the proapoptotic activity of the multikinase inhibitor BAY 43-9006. Oncogene 2005; 24: 6861–6869.
Meng XW, Lee SH, Dai H, Loegering D, Yu C, Flatten K et al. Mcl-1 as a buffer for proapoptotic Bcl-2 family members during TRAIL-induced apoptosis: a mechanistic basis for sorafenib (Bay 43-9006)-induced TRAIL sensitization. J Biol Chem 2007; 282: 29831–29846.
Zhang W, Konopleva M, Shi YX, McQueen T, Harris D, Ling X et al. Mutant FLT3: a direct target of sorafenib in acute myelogenous leukemia. J Natl Cancer Inst 2008; 100: 184–198.
Tong FK, Chow S, Hedley D . Pharmacodynamic monitoring of BAY 43-9006 (Sorafenib) in phase I clinical trials involving solid tumor and AML/MDS patients, using flow cytometry to monitor activation of the ERK pathway in peripheral blood cells. Cytometry B Clin Cytom 2006; 70: 107–114.
Metzelder S, Wang Y, Wollmer E, Wanzel M, Teichler S, Chaturvedi A et al. Compassionate use of sorafenib in FLT3-ITD-positive acute myeloid leukemia: sustained regression before and after allogeneic stem cell transplantation. Blood 2009; 113: 6567–6571.
Safaian NN, Czibere A, Bruns I, Fenk R, Reinecke P, Dienst A et al. Sorafenib (Nexavar) induces molecular remission and regression of extramedullary disease in a patient with FLT3-ITD+ acute myeloid leukemia. Leukemia Research 2009; 33: 348–350.
Strumberg D, Clark JW, Awada A, Moore MJ, Richly H, Hendlisz A et al. Safety, pharmacokinetics, and preliminary antitumor activity of sorafenib: a review of four phase I trials in patients with advanced refractory solid tumors. Oncologist 2007; 12: 426–437.
Levis M, Brown P, Smith BD, Stine A, Pham R, Stone R et al. Plasma inhibitory activity (PIA): a pharmacodynamic assay reveals insights into the basis for cytotoxic response to FLT3 inhibitors. Blood 2006; 108: 3477–3483.
Pratz KW, Cortes J, Roboz GJ, Rao N, Arowojolu O, Stine A et al. A pharmacodynamic study of the FLT3 inhibitor KW-2449 yields insight into the basis for clinical response. Blood 2009; 113: 3938–3946.
Rini BI . Sorafenib. Expert Opin Pharmacother 2006; 7: 453–461.
Lathia C, Lettieri J, Cihon F, Gallentine M, Radtke M, Sundaresan P . Lack of effect of ketoconazole-mediated CYP3A inhibition on sorafenib clinical pharmacokinetics. Cancer Chemother Pharmacol 2006; 57: 685–692.
Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH et al. Revised recommendations of the International Working Group for Diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol 2003; 21: 4642–4649.
Zhao M, Rudek MA, He P, Hafner FT, Radtke M, Wright JJ et al. A rapid and sensitive method for determination of sorafenib in human plasma using a liquid chromatography/tandem mass spectrometry assay. J Chromatogr B Analyt Technol Biomed Life Sci 2007; 846: 1–7.
Shah VP, Midha KK, Dighe S, McGilveray IJ, Skelly JP, Yacobi A et al. Analytical methods validation: bioavailability, bioequivalence and pharmacokinetic studies. Conference report. Eur J Drug Metab Pharmacokinet 1991; 16: 249–255.
Gibaldi M, Perrier D . Drug elimination and apparent volume of distribution in multicompartment systems. J Pharm Sci 1972; 61: 952–954.
Awada A, Hendlisz A, Gil T, Bartholomeus S, Mano M, de Valeriola D et al. Phase I safety and pharmacokinetics of BAY 43-9006 administered for 21 days on/7 days off in patients with advanced, refractory solid tumours. Br J Cancer 2005; 92: 1855–1861.
Clark JW, Eder JP, Ryan D, Lathia C, Lenz HJ . Safety and pharmacokinetics of the dual action Raf kinase and vascular endothelial growth factor receptor inhibitor, BAY 43-9006, in patients with advanced, refractory solid tumors. Clin Cancer Res 2005; 11: 5472–5480.
Moore M, Hirte HW, Siu L, Oza A, Hotte SJ, Petrenciuc O et al. Phase I study to determine the safety and pharmacokinetics of the novel Raf kinase and VEGFR inhibitor BAY 43-9006, administered for 28 days on/7 days off in patients with advanced, refractory solid tumors. Ann Oncol 2005; 16: 1688–1694.
Strumberg D, Richly H, Hilger RA, Schleucher N, Korfee S, Tewes M et al. Phase I clinical and pharmacokinetic study of the Novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43-9006 in patients with advanced refractory solid tumors. J Clin Oncol 2005; 23: 965–972.
Kretz O, Weiss HM, Schumacher MM, Gross G . In vitro blood distribution and plasma protein binding of the tyrosine kinase inhibitor imatinib and its active metabolite, CGP74588, in rat, mouse, dog, monkey, healthy humans and patients with acute lymphatic leukaemia. Br J Clin Pharmacol 2004; 58: 212–216.
Brown P, Levis M, Shurtleff S, Campana D, Downing J, Small D . FLT3 inhibition selectively kills childhood acute lymphoblastic leukemia cells with high levels of FLT3 expression. Blood 2005; 105: 812–820.
Stam RW, den Boer ML, Schneider P, Nollau P, Horstmann M, Beverloo HB et al. Targeting FLT3 in primary MLL-gene-rearranged infant acute lymphoblastic leukemia. Blood 2005; 106: 2484–2490.
Delmonte Jr J, Kantarjian HM, Andreeff M, Faderl S, Wright JJ, Zhang W et al. Update of a phase I study of sorafenib in patients with refractory/relapsed acute myeloid leukemia or high-risk myelodysplastic syndrome. ASH Annual Meeting Abstracts 2007; 110: 272a (abstract no. 893).
Smith BD, Levis M, Beran M, Giles F, Kantarjian H, Berg K et al. Single-agent CEP-701, a novel FLT3 inhibitor, shows biological and clinical activity in patients with relapsed or refractory acute myeloid leukemia. Blood 2004; 103: 3669–3676.
Levis M, Pham R, Smith BD, Small D . In vitro studies of a FLT3 inhibitor combined with chemotherapy: sequence of administration is important to achieve synergistic cytotoxic effects. Blood 2004; 104: 1145–1150.
Brown P, Meshinchi S, Levis M, Alonzo TA, Gerbing R, Lange B et al. Pediatric AML primary samples with FLT3/ITD mutations are preferentially killed by FLT3 inhibition. Blood 2004; 104: 1841–1849.
Pratz KW, Sato T, Murphy KM, Stine A, Rajkhowa T, Levis M . FLT3-mutant allelic burden and clinical status are predictive of response to FLT3 inhibitors in AML. Blood 2010; 115: 1425–1432.
Acknowledgements
We thank Carol Hartke, Ping He, Aleksandr Mnatsakanyan, Yelena Zabelina and Linping Xu for their technical assistance; and Susan Davidson for quality assurance of the pharmacokinetic data. This work was supported by National Institutes of Health grants P30CA006973, U01CA70095, UL1 RR025005, NCI Leukemia SPORE P50 CA100632-06, R01 CA128864 and the by American Society of Clinical Oncology (MJL). MJL is a Clinical Scholar of the Leukemia and Lymphoma Society.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Contribution: KWP designed and performed correlative assays, analyzed correlative assays, analyzed clinical trial results and wrote the paper. EC helped in analyzing correlative assays and writing the paper. MJL helped to design and interpret correlative studies and contributed patients to the study. JEK, SDG, MM each contributed to the study design, contributed patients to the study and helped to edit the paper. MAC and JJW contributed to the study design and helped to edit the paper. AS processed clinical trial specimens and helped to conduct laboratory experiments. MAR, MZ and SDB designed, conducted and interpreted the pharmacokinetic studies. BDS developed the study design and wrote the protocol, contributed patients to the study, served as the protocol PI, analyzed the clinical trial results, contributed patients to the study and helped to edit the paper.
Supplementary Information accompanies the paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Pratz, K., Cho, E., Levis, M. et al. A pharmacodynamic study of sorafenib in patients with relapsed and refractory acute leukemias. Leukemia 24, 1437–1444 (2010). https://doi.org/10.1038/leu.2010.132
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2010.132
Keywords
This article is cited by
-
Clinical outcomes in patients with relapsed/refractory FLT3-mutated acute myeloid leukemia treated with gilteritinib who received prior midostaurin or sorafenib
Blood Cancer Journal (2022)
-
Novel Therapies for Acute Myeloid Leukemia: Are We Finally Breaking the Deadlock?
Targeted Oncology (2017)
-
The Future of Targeting FLT3 Activation in AML
Current Hematologic Malignancy Reports (2017)
-
‘Acute myeloid leukemia: a comprehensive review and 2016 update’
Blood Cancer Journal (2016)
-
NFATc1 as a therapeutic target in FLT3-ITD-positive AML
Leukemia (2015)