Abstract
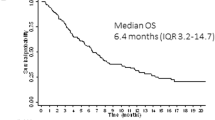
This phase 1b trial investigated several doses and schedules of midostaurin in combination with daunorubicin and cytarabine induction and high-dose cytarabine post-remission therapy in newly diagnosed patients with acute myeloid leukemia (AML). The discontinuation rate on the 50-mg twice-daily dose schedule was lower than 100 mg twice daily, and no grade 3/4 nausea or vomiting was seen. The complete remission rate for the midostaurin 50-mg twice-daily dose schedule was 80% (FMS-like tyrosine kinase 3 receptor (FLT3)–wild-type: 20 of 27 (74%), FLT3-mutant: 12 of 13 (92%)). Overall survival (OS) probabilities of patients with FLT3-mutant AML at 1 and 2 years (0.85 and 0.62, respectively) were similar to the FLT3–wild-type population (0.78 and 0.52, respectively). Midostaurin in combination with standard chemotherapy demonstrated high complete response and OS rates in newly diagnosed younger adults with AML, and was generally well tolerated at 50 mg twice daily for 14 days. A phase III prospective trial is ongoing (CALGB 10603, NCT00651261).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Thiede C, Steudel C, Mohr B, Schaich M, Schäkel U, Platzbecker U et alAnalysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood 2002; 99: 4326–4335.
Whitman SP, Archer KJ, Feng L, Baldus C, Becknell B, Carlson BD et alAbsence of the wild-type allele predicts poor prognosis in adult de novo acute myeloid leukemia with normal cytogenetics and the internal tandem duplication of FLT3: a cancer and leukemia group B study. Cancer Res 2001; 61: 7233–7239.
Weisberg E, Barrett R, Liu Q, Stone R, Gray N, Griffin JD . FLT3 inhibition and mechanisms of drug resistance in mutant FLT3-positive AML. Drug Resist Updat 2009; 12: 81–89.
Schnittger S, Schoch C, Dugas M, Kern W, Staib P, Wuchter C et alAnalysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood 2002; 100: 59–66.
Weisberg E, Boulton C, Kelly LM, Manley P, Fabbro D, Meyer T et alInhibition of mutant FLT3 receptors in leukemia cells by the small molecule tyrosine kinase inhibitor PKC412. Cancer Cell 2002; 1: 433–443.
Manley PW, Boulton C, Caravatti G, Gilliland DG, Griffin J, Kung A et alPreclinical profile of PKC412 (midostaurin) as an FLT3 inhibitor for the therapy of AML. 94th annual meeting of the American Association for Cancer Research on July 11–14, 2003 in Washington, DC. AACR 2003; Poster 1004.
Levis M, Brown P, Smith BD, Stine A, Pham R, Stone R et alPlasma inhibitory activity (PIA): a pharmacodynamic assay reveals insights into the basis for cytotoxic response to FLT3 inhibitors. Blood 2006; 108: 3477–3483.
Stone RM, DeAngelo DJ, Klimek V, Galinsky I, Estey E, Nimer SD et alPatients with acute myeloid leukemia and an activating mutation in FLT3 respond to a small-molecule FLT3 tyrosine kinase inhibitor, PKC412. Blood 2005; 105: 54–60.
Fischer T, Stone RM, DeAngelo DJ, Galinsky I, Estey E, Lanza C et alPhase IIB trial of oral midostaurin (PKC412), the FMS-like tyrosine kinase 3 receptor (FLT3) and multi-targeted kinase inhibitor, in patients with acute myeloid leukemia and high-risk myelodysplastic syndrome with either wild-type or mutated FLT3. J Clin Oncol 2010; 28: 4339–4345.
Levis M, Pham R, Smith BD, Small D . In vitro studies of a FLT3 inhibitor combined with chemotherapy: sequence of administration is important to achieve synergistic cytotoxic effects. Blood 2004; 104: 1145–1150.
Byrd JC, Mrozek K, Dodge RK, Carroll AJ, Edwards CG, Arthur DC et alPretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461). Blood 2002; 100: 4325–4336.
Ley TJ, Mardis ER, Ding L, Fulton B, McLellan MD, Chen K et alDNA sequencing of a cytogenetically normal acute myeloid leukaemia genome. Nature 2008; 456: 66–72.
Smith BD, Levis M, Beran M, Giles F, Kantarjian H, Berg K et alSingle-agent CEP-701, a novel FLT3 inhibitor, shows biologic and clinical activity in patients with relapsed or refractory acute myeloid leukemia. Blood 2004; 103: 3669–3676.
DeAngelo DJ, Stone RM, Heaney ML, Nimer SD, Paquette RL, Klisovic RB et alPhase 1 clinical results with tandutinib (MLN518), a novel FLT3 antagonist, in patients with acute myelogenous leukemia or high-risk myelodysplastic syndrome: safety, pharmacokinetics, and pharmacodynamics. Blood 2006; 108: 3674–3681.
Zhang W, Konopleva M, Shi YX, McQueen T, Harris D, Ling X et alMutant FLT3: a direct target of sorafenib in acute myelogenous leukemia. J Natl Cancer Inst 2008; 100: 184–198.
Ravandi F, Cortes JE, Jones D, Faderl S, Garcia-Manero G, Konopleva MY et alPhase I/II study of combination therapy with sorafenib, idarubicin, and cytarabine in younger patients with acute myeloid leukemia. J Clin Oncol 2010; 28: 1856–1862.
Kayser S, Schlenk RF, Londono MC, Breitenbuecher F, Wittke K, Du J et alInsertion of FLT3 internal tandem duplication in the tyrosine kinase domain-1 is associated with resistance to chemotherapy and inferior outcome. Blood 2009; 114: 2386–2392.
Breitenbuecher F, Markova B, Kasper S, Carius B, Stauder T, Böhmer FD et alA novel molecular mechanism of primary resistance to FLT3-kinase inhibitors in AML. Blood 2009; 113: 4063–4073.
Kottaridis PD, Gale RE, Frew ME, Harrison G, Langabeer SE, Belton AA et alThe presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood 2001; 98: 1752–1759.
Whitman SP, Ruppert AS, Radmacher MD, Mrozek K, Paschka P, Langer C et alFLT3 D835/I836 mutations are associated with poor disease-free survival and a distinct gene-expression signature among younger adults with de novo cytogenetically normal acute myeloid leukemia lacking FLT3 internal tandem duplications. Blood 2008; 111: 1552–1559.
Mead AJ, Linch DC, Hills RK, Wheatley K, Burnett AK, Gale RE . FLT3 tyrosine kinase domain mutations are biologically distinct from and have a significantly more favorable prognosis than FLT3 internal tandem duplications in patients with acute myeloid leukemia. Blood 2007; 110: 1262–1270.
Acknowledgements
The authors acknowledge the study coordinators, nurses and physicians who contributed to this study and the patients and their families for their participation. Susie Crowley provided secretarial assistance. Sandra Harris and Erinn Goldman of Articulate Science, LLC, provided medical writing assistance. This study was sponsored by Novartis Pharmaceuticals Corporation.
Conception and design: Richard M Stone, Thomas Fischer, Charles A Schiffer, Gerhard Ehninger, Jorge Cortes, Hagop M Kantarjian, Daniel J DeAngelo and Francis Giles. Collection and assembly of data: Richard M Stone, Ronald Paquette, Charles A Schiffer, Gerhard Ehninger, Jorge Cortes, Hagop M Kantarjian, Daniel J DeAngelo, Alice Huntsman-Labed, Catherine Dutreix, Adam del Corral and Francis Giles. Data analysis and interpretation: Richard M Stone, Thomas Fischer, Gary Schiller, Charles A Schiffer, Jorge Cortes, Hagop M Kantarjian, Daniel J DeAngelo, Alice Huntsman-Labed, Catherine Dutreix, Adam del Corral and Francis Giles. Manuscript writing: Richard M Stone wrote the first draft of the manuscript and all authors edited and commented on subsequent drafts. Final approval of manuscript: All authors gave final approval of the manuscript. Provision of study materials or patients: Richard M Stone, Thomas Fischer, Gary Schiller, Charles A Schiffer, Jorge Cortes, Daniel J DeAngelo and Francis Giles.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Employment or Leadership Position: Alice Huntsman-Labed, Novartis Pharma AG (C); Catherine Dutreix, Novartis Pharma AG (C); Adam del Corral, Novartis Pharmaceuticals Corporation (C); Consultant or Advisory Role: Richard M Stone, Genzyme (C), Celgene (C), Ariad (C); Ronald Paquette, Novartis (C); Gary Schiller, Genzme (C); Charles A Schiffer, Pfizer (C), Micromet (C), Celgene (C), Ambit (C), Ariad (C); Jorge Cortes, Novartis (C), Ariad (C), Ambit (U); Hagop M. Kantarjian, Novartis (C); Daniel J DeAngelo, Novartis (C); Francis Giles, Novartis (C); Stock Ownership: Gerhard Ehninger, Novartis; Alice Huntsman-Labed, Novartis; Honoraria: Thomas Fischer, Novartis; Ronald Paquette, Novartis; Gerhard Ehninger, Novartis; Research Funding: Richard M Stone, Novartis; Thomas Fischer, Novartis; Gary Schiller, Genzyme; Charles Schiffer, Pfizer, Ariad, Novartis, Celgene, Bristol-Myers Squibb, Ambit; Jorge Cortes, Novartis, Ariad, Ambit; Hagop M Kantarjian, Novartis, Pfizer, Bristol-Myers Squibb; Francis Giles, Novartis; Expert Testimony: None; Other Remuneration: None.
Additional information
Previous presentations: Preliminary data from this study were presented at the 51st American Society of Hematology Annual Meeting in 2009.
Rights and permissions
About this article
Cite this article
Stone, R., Fischer, T., Paquette, R. et al. Phase IB study of the FLT3 kinase inhibitor midostaurin with chemotherapy in younger newly diagnosed adult patients with acute myeloid leukemia. Leukemia 26, 2061–2068 (2012). https://doi.org/10.1038/leu.2012.115
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2012.115
Keywords
This article is cited by
-
Recent advances in targeted therapies in acute myeloid leukemia
Journal of Hematology & Oncology (2023)
-
Perspectives and challenges of small molecule inhibitor therapy for FLT3-mutated acute myeloid leukemia
Annals of Hematology (2023)
-
Infectious complications of targeted drugs and biotherapies in acute leukemia. Clinical practice guidelines by the European Conference on Infections in Leukemia (ECIL), a joint venture of the European Group for Blood and Marrow Transplantation (EBMT), the European Organization for Research and Treatment of Cancer (EORTC), the International Immunocompromised Host Society (ICHS) and the European Leukemia Net (ELN)
Leukemia (2022)
-
Adverse impact of a high allelic burden FLT3-ITD mutation on allogeneic hematopoietic stem cell transplantation in patients with cytogenetically normal AML
International Journal of Hematology (2022)
-
Which FLT3 Inhibitor for Treatment of AML?
Current Treatment Options in Oncology (2022)