Abstract
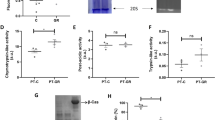
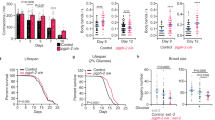
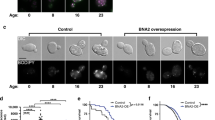
Calorie restriction extends lifespan in a broad range of organisms, from yeasts to mammals. Numerous hypotheses have been proposed to explain this phenomenon, including decreased oxidative damage and altered energy metabolism. In Saccharomyces cerevisiae, lifespan extension by calorie restriction requires the NAD+-dependent histone deacetylase, Sir2 (ref. 1). We have recently shown that Sir2 and its closest human homologue SIRT1, a p53 deacetylase, are strongly inhibited by the vitamin B3 precursor nicotinamide2. Here we show that increased expression of PNC1 (pyrazinamidase/nicotinamidase 1), which encodes an enzyme that deaminates nicotinamide, is both necessary and sufficient for lifespan extension by calorie restriction and low-intensity stress. We also identify PNC1 as a longevity gene that is responsive to all stimuli that extend lifespan. We provide evidence that nicotinamide depletion is sufficient to activate Sir2 and that this is the mechanism by which PNC1 regulates longevity. We conclude that yeast lifespan extension by calorie restriction is the consequence of an active cellular response to a low-intensity stress and speculate that nicotinamide might regulate critical cellular processes in higher organisms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lin, S. J., Defossez, P. A. & Guarente, L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science 289, 2126–2128 (2000)
Bitterman, K. J., Anderson, R. M., Cohen, H. Y., Latorre-Esteves, M. & Sinclair, D. A. Inhibition of silencing and accelerated aging by nicotinamide, a putative negative regulator of yeast Sir2 and human SIRT1. J. Biol. Chem. 277, 45099–45107 (2002)
Kaeberlein, M., Andalis, A. A., Fink, G. R. & Guarente, L. High osmolarity extends life span in Saccharomyces cerevisiae by a mechanism related to calorie restriction. Mol. Cell. Biol. 22, 8056–8066 (2002)
Jiang, J. C., Jaruga, E., Repnevskaya, M. V. & Jazwinski, S. M. An intervention resembling caloric restriction prolongs life span and retards aging in yeast. FASEB J. 14, 2135–2137 (2000)
Swiecilo, A., Krawiec, Z., Wawryn, J., Bartosz, G. & Bilinski, T. Effect of stress on the life span of the yeast Saccharomyces cerevisiae. Acta Biochim. Pol. 47, 355–364 (2000)
Smith, J. S. et al. A phylogenetically conserved NAD+-dependent protein deacetylase activity in the Sir2 protein family. Proc. Natl Acad. Sci. USA 97, 6658–6663 (2000)
Imai, S., Armstrong, C. M., Kaeberlein, M. & Guarente, L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 403, 795–800 (2000)
Tanny, J. C. & Moazed, D. Coupling of histone deacetylation to NAD breakdown by the yeast silencing protein Sir2: Evidence for acetyl transfer from substrate to an NAD breakdown product. Proc. Natl Acad. Sci. USA 98, 415–420 (2001)
Landry, J. et al. The silencing protein Sir2 and its homologs are NAD-dependent protein deacetylases. Proc. Natl Acad. Sci. USA 97, 5807–5811 (2000)
Kaeberlein, M., McVey, M. & Guarente, L. The Sir2/3/4 complex and Sir2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 13, 2570–2580 (1999)
Sinclair, D. A. & Guarente, L. Extrachromosomal rDNA circles—a cause of aging in yeast. Cell 91, 1033–1042 (1997)
Tissenbaum, H. A. & Guarente, L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature 410, 227–230 (2001)
Rogina, B., Helfand, S. L. & Frankel, S. Longevity regulation by Drosophila Rpd3 deacetylase and caloric restriction. Science 298, 1745 (2002)
Vaziri, H. et al. hSIR2(SIRT1) Functions as an NAD-dependent p53 deacetylase. Cell 107, 149–159 (2001)
Luo, J. et al. Negative control of p53 by Sir2α promotes cell survival under stress. Cell 107, 137–148 (2001)
Anderson, R. M. et al. Manipulation of a nuclear NAD+ salvage pathway delays aging without altering steady-state NAD+ levels. J. Biol. Chem. 277, 18881–18890 (2002)
Landry, J., Slama, J. T. & Sternglanz, R. Role of NAD+ in the deacetylase activity of the SIR2-like proteins. Biochem. Biophys. Res. Commun. 278, 685–690 (2000)
Gasch, A. P. et al. Genomic expression programs in the response of yeast cells to environmental changes. Mol. Biol. Cell 11, 4241–4257 (2000)
Sandmeier, J. J., Celic, I., Boeke, J. D. & Smith, J. S. Telomeric and rDNA silencing in Saccharomyces cerevisiae are dependent on a nuclear NAD+ salvage pathway. Genetics 160, 877–889 (2002)
Perichon, R., Bourre, J. M., Kelly, J. F. & Roth, G. S. The role of peroxisomes in aging. Cell Mol. Life Sci. 54, 641–652 (1998)
Lin, S. J. et al. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature 418, 344–348 (2002)
Lin, S. S., Manchester, J. K. & Gordon, J. I. Enhanced gluconeogenesis and increased energy storage as hallmarks of aging in Saccharomyces cerevisiae. J. Biol. Chem. 276, 36000–36007 (2001)
Unkefer, C. J. & London, R. E. In vivo studies of pyridine nucleotide metabolism in Escherichia coli and Saccharomyces cerevisiae by carbon-13 NMR spectroscopy. J. Biol. Chem. 259, 2311–2320 (1984)
Grant, R. S. & Kapoor, V. Murine glial cells regenerate NAD, after peroxide-induced depletion, using either nicotinic acid, nicotinamide, or quinolinic acid as substrates. J. Neurochem. 70, 1759–1763 (1998)
Aksoy, S., Szumlanski, C. L. & Weinshilboum, R. M. Human liver nicotinamide N-methyltransferase. cDNA cloning, expression, and biochemical characterization. J. Biol. Chem. 269, 14835–14840 (1994)
Niewmierzycka, A. & Clarke, S. S-Adenosylmethionine-dependent methylation in Saccharomyces cerevisiae. Identification of a novel protein arginine methyltransferase. J. Biol. Chem. 274, 814–824 (1999)
Virag, L. & Szabo, C. The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol. Rev. 54, 375–429 (2002)
Lal, A. et al. A public database for gene expression in human cancers. Cancer Res. 59, 5403–5407 (1999)
Kassem, H., Sangar, V., Cowan, R., Clarke, N. & Margison, G. P. A potential role of heat shock proteins and nicotinamide N-methyl transferase in predicting response to radiation in bladder cancer. Int. J. Cancer 101, 454–460 (2002)
Ghislain, M., Talla, E. & Francois, J. M. Identification and functional analysis of the Saccharomyces cerevisiae nicotinamidase gene, PNC1. Yeast 19, 215–224 (2002)
Acknowledgements
We thank members of the Sinclair laboratory, R. Veech, C. Wolberger, W. Forrester, S. Luikenhuis and D. Finkelstein, for reagents and discussions. This work was supported by the NIA and the Harvard–Armenise Foundation. D.S. is an Ellison Medical Research Foundation Special Fellow. R.A. is supported by a John Taplan Postdoctoral Fellowship, J.W. by a National Science Foundation Scholarship, and K.B. and O.M. by the American Federation of Aging Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Rights and permissions
About this article
Cite this article
Anderson, R., Bitterman, K., Wood, J. et al. Nicotinamide and PNC1 govern lifespan extension by calorie restriction in Saccharomyces cerevisiae. Nature 423, 181–185 (2003). https://doi.org/10.1038/nature01578
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature01578
This article is cited by
-
The impacts of nicotinamide and inositol on the available cells and product performance of industrial baker's yeasts
Bioresources and Bioprocessing (2023)
-
Mechanisms, pathways and strategies for rejuvenation through epigenetic reprogramming
Nature Aging (2023)
-
Multiple myeloma, a quintessential malignant disease of aging: a geroscience perspective on pathogenesis and treatment
GeroScience (2023)
-
Dietary regulation in health and disease
Signal Transduction and Targeted Therapy (2022)
-
The flavonoid corylin exhibits lifespan extension properties in mouse
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.