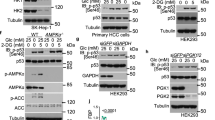
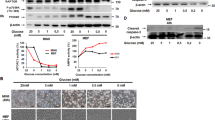
Abstract
Glycolysis and apoptosis are considered major but independent pathways that are critical for cell survival1,2,3,4. The activity of BAD, a pro-apoptotic BCL-2 family member, is regulated by phosphorylation in response to growth/survival factors5,6,7,8. Here we undertook a proteomic analysis to assess whether BAD might also participate in mitochondrial physiology. In liver mitochondria, BAD resides in a functional holoenzyme complex together with protein kinase A7 and protein phosphatase 1 (PP1) catalytic units9, Wiskott–Aldrich family member WAVE-1 as an A kinase anchoring protein10, and glucokinase (hexokinase IV)11. BAD is required to assemble the complex in that Bad-deficient hepatocytes lack this complex, resulting in diminished mitochondria-based glucokinase activity and blunted mitochondrial respiration in response to glucose. Glucose deprivation results in dephosphorylation of BAD, and BAD-dependent cell death. Moreover, the phosphorylation status of BAD helps regulate glucokinase activity. Mice deficient for BAD or bearing a non-phosphorylatable BAD(3SA) mutant12 display abnormal glucose homeostasis including profound defects in glucose tolerance. This combination of proteomics, genetics and physiology indicates an unanticipated role for BAD in integrating pathways of glucose metabolism and apoptosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Raff, M. C. Social controls on cell survival and cell death. Nature 356, 397–400 (1992)
Vander Heiden, M. G. et al. Growth factors can influence cell growth and survival through effects on glucose metabolism. Mol. Cell. Biol. 21, 5899–5912 (2001)
Gottlob, K. et al. Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes Dev. 15, 1406–1418 (2001)
Wang, X. The expanding role of mitochondria in apoptosis. Genes Dev. 15, 2922–2933 (2001)
Zha, J., Harada, H., Yang, E., Jockel, J. & Korsmeyer, S. J. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L). Cell 87, 619–628 (1996)
Datta, S. R. et al. 14-3-3 proteins and survival kinases cooperate to inactivate BAD by BH3 domain phosphorylation. Mol. Cell 6, 41–51 (2000)
Harada, H. et al. Phosphorylation and inactivation of BAD by mitochondria-anchored protein kinase A. Mol. Cell 3, 413–422 (1999)
Harada, H., Andersen, J. S., Mann, M., Terada, N. & Korsmeyer, S. J. p70S6 kinase signals cell survival as well as growth, inactivating the pro-apoptotic molecule BAD. Proc. Natl Acad. Sci. USA 98, 9666–9670 (2001)
Moorhead, G., MacKintosh, C., Morrice, N. & Cohen, P. Purification of the hepatic glycogen-associated form of protein phosphatase-1 by microcystin-Sepharose affinity chromatography. FEBS Lett. 362, 101–105 (1995)
Westphal, R. S., Soderling, S. H., Alto, N. M., Langeberg, L. K. & Scott, J. D. Scar/WAVE-1, a Wiskott-Aldrich syndrome protein, assembles an actin-associated multi-kinase scaffold. EMBO J. 19, 4589–4600 (2000)
Postic, C., Shiota, M. & Magnuson, M. A. Cell-specific roles of glucokinase in glucose homeostasis. Recent Prog. Horm. Res. 56, 195–217 (2001)
Datta, S. R. et al. Survival factor-mediated BAD phosphorylation raises the mitochondrial threshold for apoptosis. Dev. Cell 3, 631–643 (2002)
Wei, M. C. et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292, 727–730 (2001)
Cheng, E. H. et al. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol. Cell 8, 705–711 (2001)
Hsu, Y. T. & Youle, R. J. Bax in murine thymus is a soluble monomeric protein that displays differential detergent-induced conformations. J. Biol. Chem. 273, 10777–10783 (1998)
Krimmer, T. et al. Biogenesis of porin of the outer mitochondrial membrane involves an import pathway via receptors and the general import pore of the TOM complex. J. Cell Biol. 152, 289–300 (2001)
Abdul, K. M. et al. Functional analysis of human metaxin in mitochondrial protein import in cultured cells and its relationship with the Tom complex. Biochem. Biophys. Res. Commun. 276, 1028–1034 (2000)
Ayllon, V., Martinez, A. C., Garcia, A., Cayla, X. & Rebollo, A. Protein phosphatase 1α is a Ras-activated Bad phosphatase that regulates interleukin-2 deprivation-induced apoptosis. EMBO J. 19, 2237–2246 (2000)
Westphal, R. S. et al. Regulation of NMDA receptors by an associated phosphatase-kinase signaling complex. Science 285, 93–96 (1999)
Machesky, L. M. & Insall, R. H. Scar1 and the related Wiskott-Aldrich syndrome protein, WASP, regulate the actin cytoskeleton through the Arp2/3 complex. Curr. Biol. 8, 1347–1356 (1998)
Postic, C. et al. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic beta cell-specific gene knock-outs using Cre recombinase. J. Biol. Chem. 274, 305–315 (1999)
Bali, D. et al. Animal model for maturity-onset diabetes of the young generated by disruption of the mouse glucokinase gene. J. Biol. Chem. 270, 21464–21467 (1995)
Cho, H. et al. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta). Science 292, 1728–1731 (2001)
Plas, D. R., Talapatra, S., Edinger, A. L., Rathmell, J. C. & Thompson, C. B. Akt and Bcl-xL promote growth factor-independent survival through distinct effects on mitochondrial physiology. J. Biol. Chem. 276, 12041–12048 (2001)
Garland, J. M. & Halestrap, A. Energy metabolism during apoptosis. Bcl-2 promotes survival in hematopoietic cells induced to apoptose by growth factor withdrawal by stabilizing a form of metabolic arrest. J. Biol. Chem. 272, 4680–4688 (1997)
Murata, T. et al. Co-localization of glucokinase with actin filaments. FEBS Lett. 406, 109–113 (1997)
Dekker, P. J. et al. The Tim core complex defines the number of mitochondrial translocation contact sites and can hold arrested preproteins in the absence of matrix Hsp70-Tim44. EMBO J. 16, 5408–5419 (1997)
Licklider, L. J., Thoreen, C. C., Peng, J. & Gygi, S. P. Automation of nanoscale microcapillary liquid chromatography-tandem mass spectrometry with a vented column. Anal. Chem. 74, 3076–3083 (2002)
Niswender, K. D. et al. Cell-specific expression and regulation of a glucokinase gene locus transgene. J. Biol. Chem. 272, 22564–22569 (1997)
Zhang, C. Y. et al. Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction, and type 2 diabetes. Cell 105, 745–755 (2001)
Acknowledgements
We thank M. Ryan, G. Shore, J. Scott, M. Magnuson and B. Spiegelman for reagents; M. Ryan and J. Opferman for technical advice; B. Kahn and O. Peroni for discussion; S. Wade, J. Fisher and J. Sturgill for animal care; U. Maduekwe for technical assistance; and E. Smith for manuscript preparation. N.N.D. is a recipient of the Cancer Research Fund of Damon Runyon Foundation fellowship. This work is supported in part by a NIH grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Danial, N., Gramm, C., Scorrano, L. et al. BAD and glucokinase reside in a mitochondrial complex that integrates glycolysis and apoptosis. Nature 424, 952–956 (2003). https://doi.org/10.1038/nature01825
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature01825
This article is cited by
-
Dual role of PID1 in regulating apoptosis induced by distinct anticancer-agents through AKT/Raf-1-dependent pathway in hepatocellular carcinoma
Cell Death Discovery (2023)
-
Liver-specific overexpression of HKDC1 increases hepatocyte size and proliferative capacity
Scientific Reports (2023)
-
Venlafaxine’s effect on resilience to stress is associated with a shift in the balance between glucose and fatty acid utilization
Neuropsychopharmacology (2023)
-
The multiple links between actin and mitochondria
Nature Reviews Molecular Cell Biology (2023)
-
Non-metabolic role of alpha-enolase in virus replication
Molecular Biology Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.