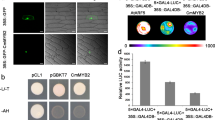
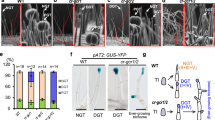
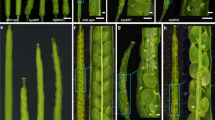
Abstract
Plants with altered microRNA metabolism have pleiotropic developmental defects, but direct evidence for microRNAs regulating specific aspects of plant morphogenesis has been lacking. In a genetic screen, we identified the JAW locus, which produces a microRNA that can guide messenger RNA cleavage of several TCP genes controlling leaf development. MicroRNA-guided cleavage of TCP4 mRNA is necessary to prevent aberrant activity of the TCP4 gene expressed from its native promoter. In addition, overexpression of wild-type and microRNA-resistant TCP variants demonstrates that mRNA cleavage is largely sufficient to restrict TCP function to its normal domain of activity. TCP genes with microRNA target sequences are found in a wide range of species, indicating that microRNA-mediated control of leaf morphogenesis is conserved in plants with very different leaf forms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Nath, U., Crawford, B. C., Carpenter, R. & Coen, E. Genetic control of surface curvature. Science 299, 1404–1407 (2003)
Cubas, P., Lauter, N., Doebley, J. & Coen, E. The TCP domain: a motif found in proteins regulating plant growth and development. Plant J. 18, 215–222 (1999)
Llave, C., Xie, Z., Kasschau, K. D. & Carrington, J. C. Cleavage of Scarecrow-like mRNA targets directed by a class of Arabidopsis miRNA. Science 297, 2053–2056 (2002)
Tang, G., Reinhart, B. J., Bartel, D. P. & Zamore, P. D. A biochemical framework for RNA silencing in plants. Genes Dev. 17, 49–63 (2003)
Plasterk, R. H. RNA silencing: the genome's immune system. Science 296, 1263–1265 (2002)
Hutvágner, G. et al. A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science 293, 834–838 (2001)
Reinhart, B. J., Weinstein, E. G., Rhoades, M. W., Bartel, B. & Bartel, D. P. MicroRNAs in plants. Genes Dev. 16, 1616–1626 (2002)
Carmell, M. A., Xuan, Z., Zhang, M. Q. & Hannon, G. J. The Argonaute family: tentacles that reach into RNAi, developmental control, stem cell maintenance, and tumorigenesis. Genes Dev. 16, 2733–2742 (2002)
Park, W., Li, J., Song, R., Messing, J. & Chen, X. CARPEL FACTORY, a Dicer homolog, and HEN1, a novel protein, act in microRNA metabolism in Arabidopsis thaliana. Curr. Biol. 12, 1484–1495 (2002)
Jacobsen, S. E., Running, M. P. & Meyerowitz, E. M. Disruption of an RNA helicase/RNAse III gene in Arabidopsis causes unregulated cell division in floral meristems. Development 126, 5231–5243 (1999)
Golden, T. A. et al. SHORT INTEGUMENTS1/SUSPENSOR1/CARPEL FACTORY, a Dicer homolog, is a maternal effect gene required for embryo development in Arabidopsis. Plant Physiol. 130, 808–822 (2002)
Kasschau, K. D. et al. P1/HC-Pro, a viral suppressor of RNA silencing, interferes with Arabidopsis development and miRNA function. Dev. Cell 4, 205–217 (2003)
Xie, Z., Kasschau, K. D. & Carrington, J. C. Negative feedback regulation of Dicer-Like1 in Arabidopsis by microRNA-guided mRNA degradation. Curr. Biol. 13, 784–789 (2003)
Weigel, D. et al. Activation tagging in Arabidopsis. Plant Physiol. 122, 1003–1013 (2000)
Mallory, A. C., Reinhart, B. J., Bartel, D., Vance, V. B. & Bowman, L. H. A viral suppressor of RNA silencing differentially regulates the accumulation of short interfering RNAs and micro-RNAs in tobacco. Proc. Natl Acad. Sci. USA 99, 15228–15233 (2002)
Elbashir, S. M., Martinez, J., Patkaniowska, A., Lendeckel, W. & Tuschl, T. Functional anatomy of siRNAs for mediating efficient RNAi in Drosophila melanogaster embryo lysate. EMBO J. 20, 6877–6888 (2001)
Rhoades, M. W. et al. Prediction of plant microRNA targets. Cell 110, 513–520 (2002)
Mayer, U., Torres Ruiz, R. A., Berleth, T., Miséra, S. & Jürgens, G. Mutations affecting body organization in the Arabidopsis embryo. Nature 353, 402–407 (1991)
Aida, M., Ishida, T., Fukaki, H., Fujisawa, H. & Tasaka, M. Genes involved in organ separation in Arabidopsis: an analysis of the cup-shaped cotyledon mutant. Plant Cell 9, 841–857 (1997)
Hadfi, K., Speth, V. & Neuhaus, G. Auxin-induced developmental patterns in Brassica juncea embryos. Development 125, 879–887 (1998)
Hamilton, A. J. & Baulcombe, D. C. A species of small antisense RNA in posttranscriptional gene silencing in plants. Science 286, 950–952 (1999)
Hammond, S. M., Bernstein, E., Beach, D. & Hannon, G. J. An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature 404, 293–296 (2000)
Lee, R. C., Feinbaum, R. L. & Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75, 843–854 (1993)
Wightman, B., Ha, I. & Ruvkun, G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 75, 855–862 (1993)
Reinhart, B. J. et al. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature 403, 901–906 (2000)
Brennecke, J., Hipfner, D. R., Stark, A., Russell, R. B. & Cohen, S. M. bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell 113, 25–36 (2003)
Kawasaki, H. & Taira, K. Hes1 is a target of microRNA-23 during retinoic-acid-induced neuronal differentiation of NT2 cells. Nature 423, 838–842 (2003)
Hutvágner, G. & Zamore, P. D. A microRNA in a multiple-turnover RNAi enzyme complex. Science 297, 2056–2060 (2002)
McConnell, J. R. et al. Role of PHABULOSA and PHAVOLUTA in determining radial patterning in shoots. Nature 411, 709–713 (2001)
Weigel, D. & Glazebrook, J. Arabidopsis: A Laboratory Manual (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, 2002)
Irizarry, R. A. et al. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 31, e15, doi:10.1093/nar/gng015 (2003)
Heid, C. A., Stevens, J., Livak, K. J. & Williams, P. M. Real time quantitative PCR. Genome Res. 6, 986–994 (1996)
Smyth, D. R., Bowman, J. L. & Meyerowitz, E. M. Early flower development in Arabidopsis. Plant Cell 2, 755–767 (1990)
Thompson, J. D., Gibson, T. J., Plewniak, F., Jeanmougin, F. & Higgins, D. G. The CLUSTAL-X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25, 4876–4882 (1997)
Swofford, D. L. PAUP: A computer program for phylogenetic inference using maximum parsimony. Gen. Physiol. 102, 9A (1993)
Huelsenbeck, J. P. & Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17, 754–755 (2001)
Rozas, J. & Rozas, R. DnaSP version 3: an integrated program for molecular population genetics and molecular evolution analysis. Bioinformatics 15, 174–175 (1999)
Citerne, H. L., Luo, D., Pennington, R. T., Coen, E. & Cronk, Q. C. A phylogenomic investigation of CYCLOIDEA-like TCP genes in the Leguminosae. Plant Physiol. 131, 1042–1053 (2003)
Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 31, 3406–3415 (2003)
Chen, X. A microRNA as a translational repressor of APETALA2 in Arabidopsis flower development. Science published online 31 July 2003 (doi:10.1126/science.1088060)
Acknowledgements
We thank I. Puga-Gonzalez for assistance and V. Ambros, S. Balasubramanian, J. Chory, M.-C. Kim, J. Lohmann, Y. Kobayashi and J. Spatafora for advice and discussion. This work was supported by fellowships from CONICET and Human Frontier Science Program Organization to J.F.P., from Life Sciences Research Foundation/US Department of Energy to X.W.; by grants from NSF and NIH to J.C.C. and NIH to D.W.; and by the Max Planck Society. D.W. is a Director of the Max Planck Institute.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Palatnik, J., Allen, E., Wu, X. et al. Control of leaf morphogenesis by microRNAs. Nature 425, 257–263 (2003). https://doi.org/10.1038/nature01958
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature01958
This article is cited by
-
Genomic survey, bioinformatics analysis, and expression profiles of TCP genes in Liriodendron chinense and functional characterization of LcTCP4
Trees (2024)
-
A first look at sea-lavenders genomics – can genome wide SNP information tip the scales of controversy in the Limonium vulgare species complex?
BMC Plant Biology (2023)
-
Genome-wide identification and analysis of TCP family genes in Medicago sativa reveal their critical roles in Na+/K+ homeostasis
BMC Plant Biology (2023)
-
An optimized pipeline for live imaging whole Arabidopsis leaves at cellular resolution
Plant Methods (2023)
-
The cell cycle arrested results in the premature advent of apical leaflets development cessation in Zygophyllum xanthoxylon
Trees (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.